Abstract
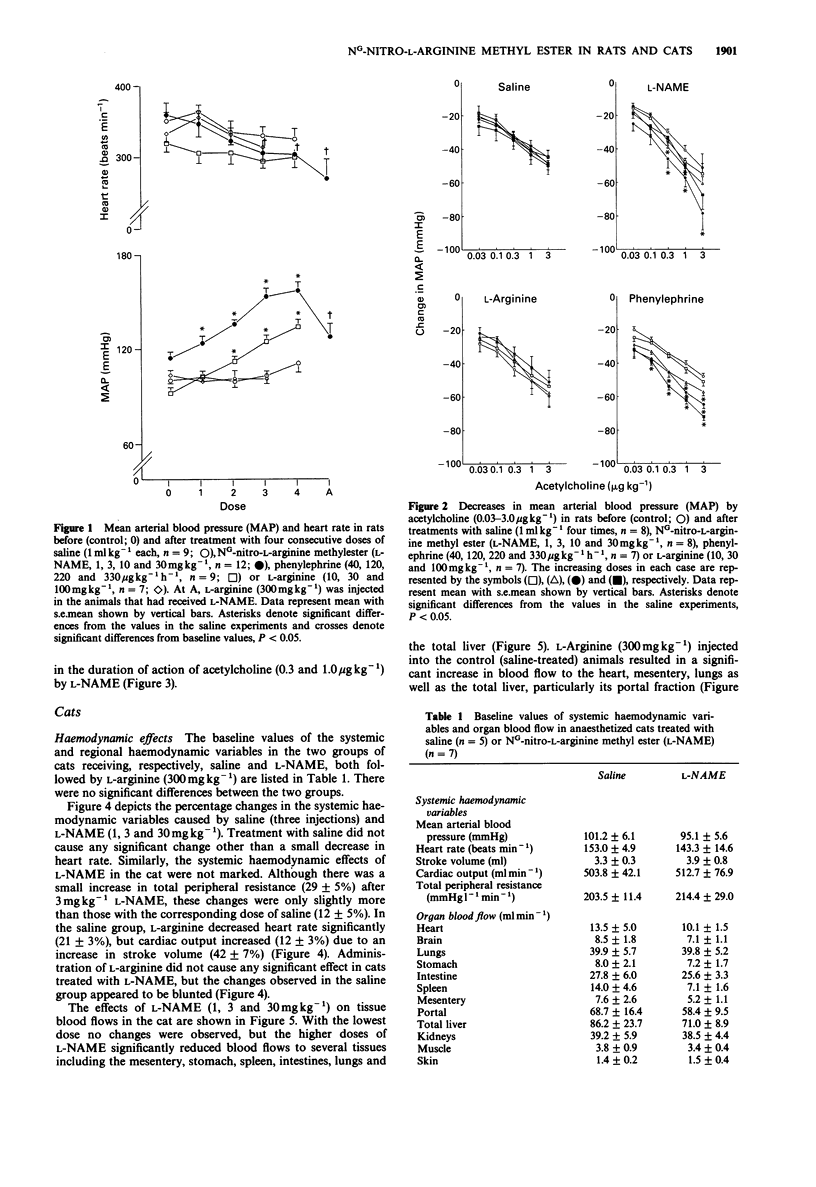
1. The haemodynamic effects of NG-nitro-L-arginine methylester (L-NAME; 1, 3, 10 and 30 mg kg-1) and its potential ability to attenuate the hypotensive responses to acetylcholine (0.03, 0.1, 1.0 and 3.0 micrograms kg-1) have been investigated in anaesthetized rats and cats. 2. In the rat, L-NAME elicited a dose-dependent pressor effect increasing mean arterial blood pressure from the baseline value of 116 +/- 4 mmHg to a maximum of 156 +/- 6 mmHg with 30 mg kg-1. This increase in blood pressure could be only partly reversed by L-arginine (300 mg kg-1). However, the increase in blood pressure by lower doses (up to 10 mg kg-1) of L-NAME was effectively reversed by L-arginine (1000 mg kg-1). 3. In the cat, L-NAME did not significantly modify systemic haemodynamic variables (heart rate, mean arterial blood pressure, cardiac output, stroke volume or total peripheral resistance), when compared to the changes in saline-treated animals. Administration of L-arginine did not cause any significant effect in cats treated with L-NAME, but some decrease in heart rate and increases in cardiac output and stroke volume were observed in the saline-treated group. 4. With the lowest dose (1 mg kg-1), L-NAME did not affect tissue blood flows in the cat, but higher doses (3 and 30 mg kg-1) significantly reduced blood flows to the mesentery, stomach, spleen, intestines, lungs and the total liver.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF

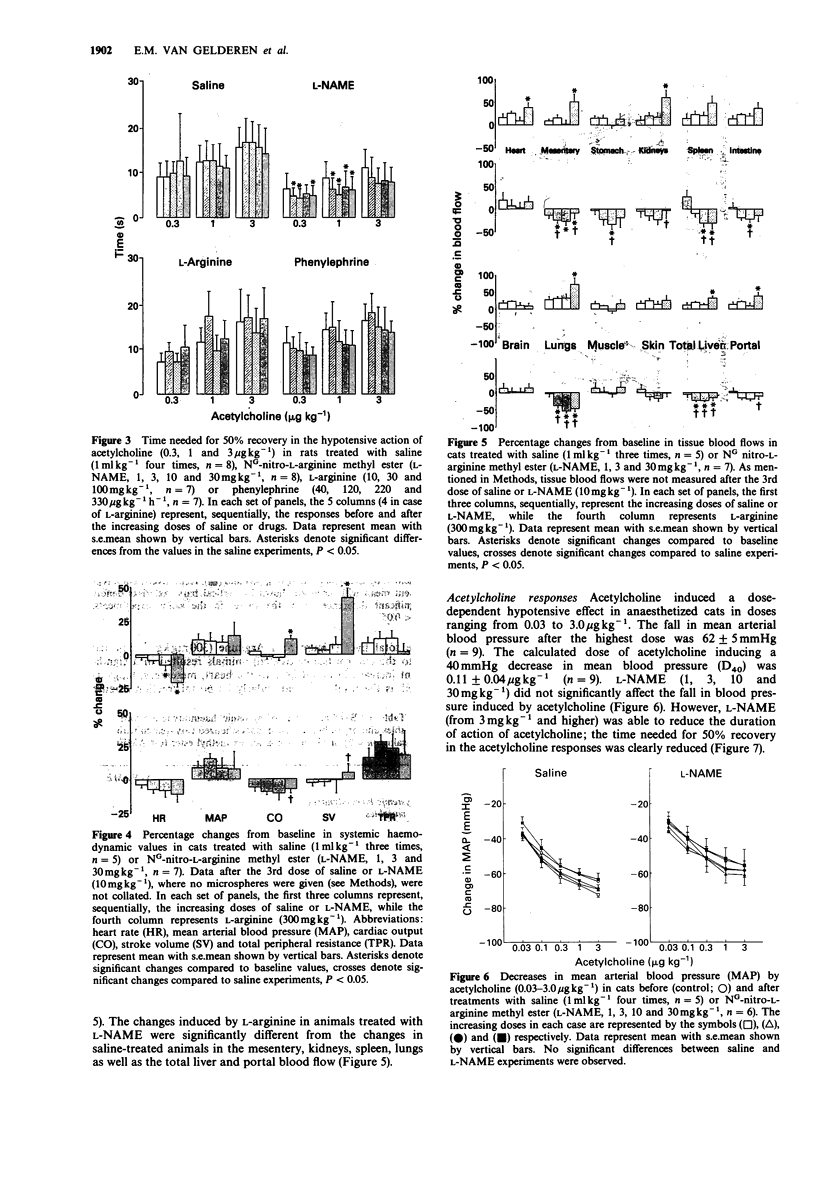


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aisaka K., Gross S. S., Griffith O. W., Levi R. L-arginine availability determines the duration of acetylcholine-induced systemic vasodilation in vivo. Biochem Biophys Res Commun. 1989 Sep 15;163(2):710–717. doi: 10.1016/0006-291x(89)92281-x. [DOI] [PubMed] [Google Scholar]
- Boulanger C., Schini V. B., Moncada S., Vanhoutte P. M. Stimulation of cyclic GMP production in cultured endothelial cells of the pig by bradykinin, adenosine diphosphate, calcium ionophore A23187 and nitric oxide. Br J Pharmacol. 1990 Sep;101(1):152–156. doi: 10.1111/j.1476-5381.1990.tb12105.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furchgott R. F., Vanhoutte P. M. Endothelium-derived relaxing and contracting factors. FASEB J. 1989 Jul;3(9):2007–2018. [PubMed] [Google Scholar]
- Gardiner S. M., Compton A. M., Bennett T., Palmer R. M., Moncada S. Regional haemodynamic changes during oral ingestion of NG-monomethyl-L-arginine or NG-nitro-L-arginine methyl ester in conscious Brattleboro rats. Br J Pharmacol. 1990 Sep;101(1):10–12. doi: 10.1111/j.1476-5381.1990.tb12079.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardiner S. M., Compton A. M., Kemp P. A., Bennett T. Regional and cardiac haemodynamic effects of NG-nitro-L-arginine methyl ester in conscious, Long Evans rats. Br J Pharmacol. 1990 Nov;101(3):625–631. doi: 10.1111/j.1476-5381.1990.tb14131.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardiner S. M., Compton A. M., Kemp P. A., Bennett T. Regional and cardiac haemodynamic responses to glyceryl trinitrate, acetylcholine, bradykinin and endothelin-1 in conscious rats: effects of NG-nitro-L-arginine methyl ester. Br J Pharmacol. 1990 Nov;101(3):632–639. doi: 10.1111/j.1476-5381.1990.tb14132.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heymann M. A., Payne B. D., Hoffman J. I., Rudolph A. M. Blood flow measurements with radionuclide-labeled particles. Prog Cardiovasc Dis. 1977 Jul-Aug;20(1):55–79. doi: 10.1016/s0033-0620(77)80005-4. [DOI] [PubMed] [Google Scholar]
- Humphries R. G., Carr R. D., Nicol A. K., Tomlinson W., O'Connor S. E. Coronary vasoconstriction in the conscious rabbit following intravenous infusion of L-NG-nitro-arginine. Br J Pharmacol. 1991 Mar;102(3):565–566. doi: 10.1111/j.1476-5381.1991.tb12212.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelm M., Schrader J. Control of coronary vascular tone by nitric oxide. Circ Res. 1990 Jun;66(6):1561–1575. doi: 10.1161/01.res.66.6.1561. [DOI] [PubMed] [Google Scholar]
- Moore P. K., al-Swayeh O. A., Chong N. W., Evans R. A., Gibson A. L-NG-nitro arginine (L-NOARG), a novel, L-arginine-reversible inhibitor of endothelium-dependent vasodilatation in vitro. Br J Pharmacol. 1990 Feb;99(2):408–412. doi: 10.1111/j.1476-5381.1990.tb14717.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mülsch A., Busse R. NG-nitro-L-arginine (N5-[imino(nitroamino)methyl]-L-ornithine) impairs endothelium-dependent dilations by inhibiting cytosolic nitric oxide synthesis from L-arginine. Naunyn Schmiedebergs Arch Pharmacol. 1990 Jan-Feb;341(1-2):143–147. doi: 10.1007/BF00195071. [DOI] [PubMed] [Google Scholar]
- Palmer R. M., Ashton D. S., Moncada S. Vascular endothelial cells synthesize nitric oxide from L-arginine. Nature. 1988 Jun 16;333(6174):664–666. doi: 10.1038/333664a0. [DOI] [PubMed] [Google Scholar]
- Palmer R. M., Ferrige A. G., Moncada S. Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor. Nature. 1987 Jun 11;327(6122):524–526. doi: 10.1038/327524a0. [DOI] [PubMed] [Google Scholar]
- Rees D. D., Palmer R. M., Schulz R., Hodson H. F., Moncada S. Characterization of three inhibitors of endothelial nitric oxide synthase in vitro and in vivo. Br J Pharmacol. 1990 Nov;101(3):746–752. doi: 10.1111/j.1476-5381.1990.tb14151.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxena P. R., Schamhardt H. C., Forsyth R. P., Hoeve J. Computer programs for the radioactive microsphere technique. Determination of regional blood flows and other haemodynamic variables in different experimental circumstances. Comput Programs Biomed. 1980 Dec;12(2-3):63–84. doi: 10.1016/0010-468x(80)90053-7. [DOI] [PubMed] [Google Scholar]
- Tare M., Parkington H. C., Coleman H. A., Neild T. O., Dusting G. J. Hyperpolarization and relaxation of arterial smooth muscle caused by nitric oxide derived from the endothelium. Nature. 1990 Jul 5;346(6279):69–71. doi: 10.1038/346069a0. [DOI] [PubMed] [Google Scholar]
- Vallance P., Collier J., Moncada S. Nitric oxide synthesised from L-arginine mediates endothelium dependent dilatation in human veins in vivo. Cardiovasc Res. 1989 Dec;23(12):1053–1057. doi: 10.1093/cvr/23.12.1053. [DOI] [PubMed] [Google Scholar]
- Whittle B. J., Lopez-Belmonte J., Rees D. D. Modulation of the vasodepressor actions of acetylcholine, bradykinin, substance P and endothelin in the rat by a specific inhibitor of nitric oxide formation. Br J Pharmacol. 1989 Oct;98(2):646–652. doi: 10.1111/j.1476-5381.1989.tb12639.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


