Abstract
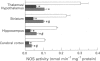
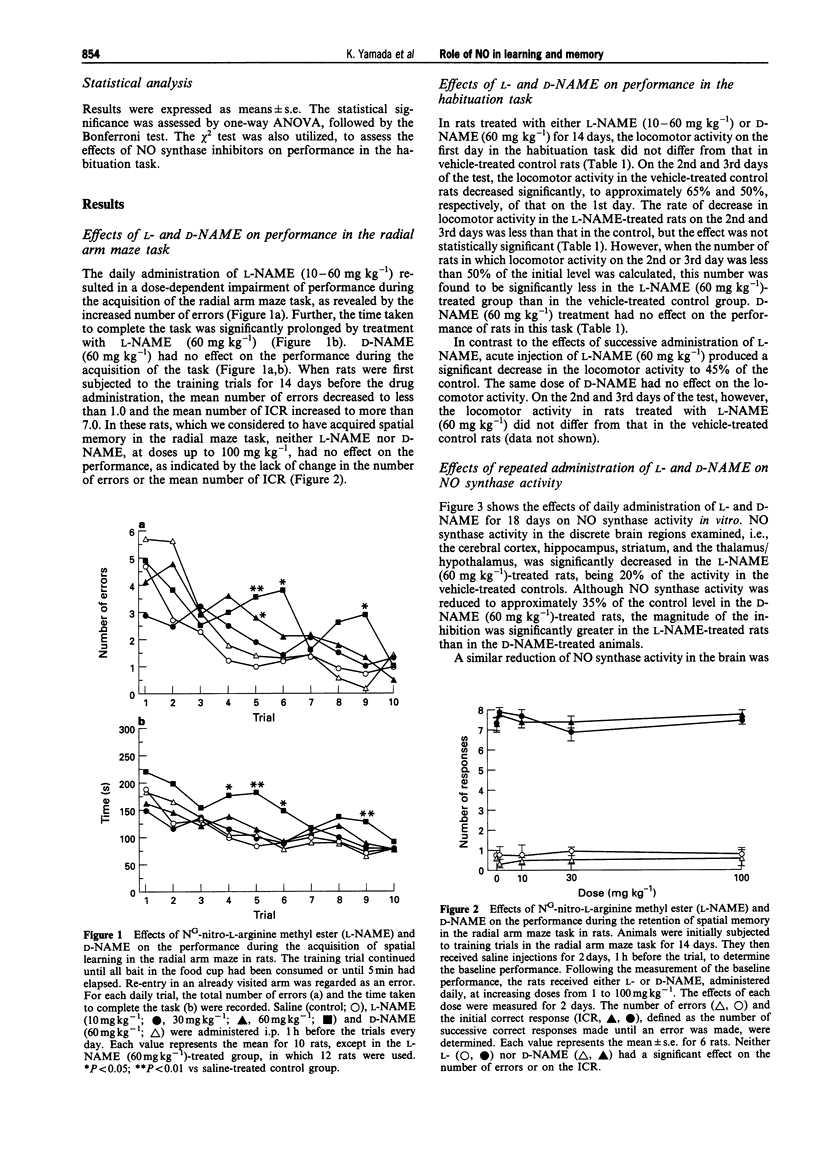
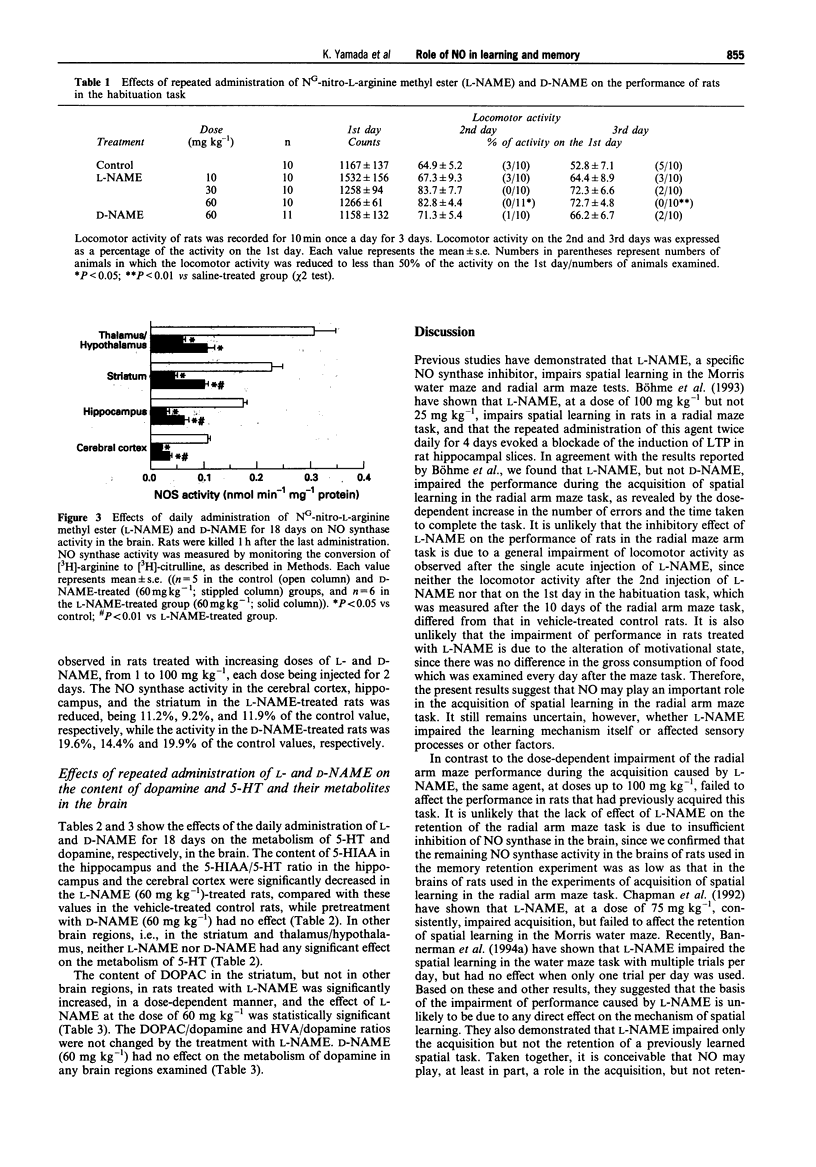
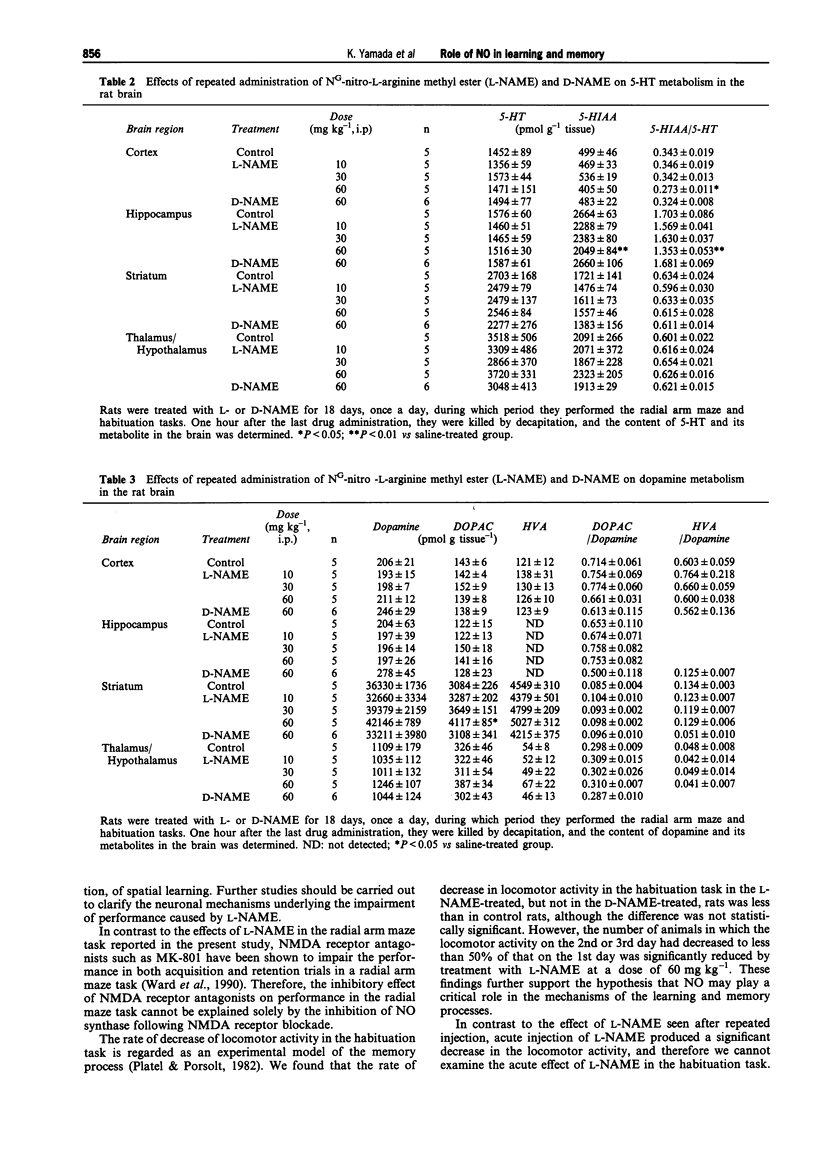
1. We investigated the effects of NG-nitro-L-arginine methyl ester (L-NAME), an inhibitor of nitric oxide (NO) synthase, on the performance of rats in a radial arm maze and in habituation tasks, and on monoamine metabolism in the brain. 2. Daily administration of L-NAME (10-60 mg kg-1) resulted in a dose-dependent impairment of performance during the acquisition of the radial arm maze task, while it failed to affect performance in those rats that had previously acquired the task. 3. The rate of decrease in locomotor activity in the habituation task in the L-NAME-treated rats was significantly less than that in control rats. 4. NG-nitro-D-arginine methyl ester (D-NAME, a less active inhibitor of NO synthase) showed no effects in the above behavioural tasks. 5. NO synthase activity was significantly decreased in both the L-NAME and D-NAME-treated rats, with the magnitude of inhibition being greater in the L-NAME-treated animals. 6. The content of 5-hydroxyindoleacetic acid (5-HIAA) in the hippocampus and the 5-HIAA/5-hydroxytryptamine ratio in the hippocampus and cortex were significantly decreased in the L-NAME (60 mg kg-1)-treated rats compared with these values in the controls. 7. Striatal 3,4-dihydroxyphenylacetic acid (DOPAC) content was significantly increased in the L-NAME (60 mg kg-1)-treated rats compared with the values in the controls, while the DOPAC/dopamine ratio was not changed.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bannerman D. M., Chapman P. F., Kelly P. A., Butcher S. P., Morris R. G. Inhibition of nitric oxide synthase does not impair spatial learning. J Neurosci. 1994 Dec;14(12):7404–7414. doi: 10.1523/JNEUROSCI.14-12-07404.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bannerman D. M., Chapman P. F., Kelly P. A., Butcher S. P., Morris R. G. Inhibition of nitric oxide synthase does not prevent the induction of long-term potentiation in vivo. J Neurosci. 1994 Dec;14(12):7415–7425. doi: 10.1523/JNEUROSCI.14-12-07415.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bredt D. S., Snyder S. H. Nitric oxide mediates glutamate-linked enhancement of cGMP levels in the cerebellum. Proc Natl Acad Sci U S A. 1989 Nov;86(22):9030–9033. doi: 10.1073/pnas.86.22.9030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bredt D. S., Snyder S. H. Nitric oxide, a novel neuronal messenger. Neuron. 1992 Jan;8(1):3–11. doi: 10.1016/0896-6273(92)90104-l. [DOI] [PubMed] [Google Scholar]
- Böhme G. A., Bon C., Lemaire M., Reibaud M., Piot O., Stutzmann J. M., Doble A., Blanchard J. C. Altered synaptic plasticity and memory formation in nitric oxide synthase inhibitor-treated rats. Proc Natl Acad Sci U S A. 1993 Oct 1;90(19):9191–9194. doi: 10.1073/pnas.90.19.9191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldwell M., O'Neill M., Earley B., Leonard B. NG-Nitro-L-arginine protects against ischaemia-induced increases in nitric oxide and hippocampal neuro-degeneration in the gerbil. Eur J Pharmacol. 1994 Aug 1;260(2-3):191–200. doi: 10.1016/0014-2999(94)90337-9. [DOI] [PubMed] [Google Scholar]
- Chapman P. F., Atkins C. M., Allen M. T., Haley J. E., Steinmetz J. E. Inhibition of nitric oxide synthesis impairs two different forms of learning. Neuroreport. 1992 Jul;3(7):567–570. doi: 10.1097/00001756-199207000-00005. [DOI] [PubMed] [Google Scholar]
- Dawson T. M., Snyder S. H. Gases as biological messengers: nitric oxide and carbon monoxide in the brain. J Neurosci. 1994 Sep;14(9):5147–5159. doi: 10.1523/JNEUROSCI.14-09-05147.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson V. L., Dawson T. M., Bartley D. A., Uhl G. R., Snyder S. H. Mechanisms of nitric oxide-mediated neurotoxicity in primary brain cultures. J Neurosci. 1993 Jun;13(6):2651–2661. doi: 10.1523/JNEUROSCI.13-06-02651.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson V. L., Dawson T. M., London E. D., Bredt D. S., Snyder S. H. Nitric oxide mediates glutamate neurotoxicity in primary cortical cultures. Proc Natl Acad Sci U S A. 1991 Jul 15;88(14):6368–6371. doi: 10.1073/pnas.88.14.6368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dwyer M. A., Bredt D. S., Snyder S. H. Nitric oxide synthase: irreversible inhibition by L-NG-nitroarginine in brain in vitro and in vivo. Biochem Biophys Res Commun. 1991 May 15;176(3):1136–1141. doi: 10.1016/0006-291x(91)90403-t. [DOI] [PubMed] [Google Scholar]
- East S. J., Garthwaite J. NMDA receptor activation in rat hippocampus induces cyclic GMP formation through the L-arginine-nitric oxide pathway. Neurosci Lett. 1991 Feb 11;123(1):17–19. doi: 10.1016/0304-3940(91)90147-l. [DOI] [PubMed] [Google Scholar]
- Garthwaite J., Charles S. L., Chess-Williams R. Endothelium-derived relaxing factor release on activation of NMDA receptors suggests role as intercellular messenger in the brain. Nature. 1988 Nov 24;336(6197):385–388. doi: 10.1038/336385a0. [DOI] [PubMed] [Google Scholar]
- Garthwaite J., Garthwaite G., Palmer R. M., Moncada S. NMDA receptor activation induces nitric oxide synthesis from arginine in rat brain slices. Eur J Pharmacol. 1989 Oct 17;172(4-5):413–416. doi: 10.1016/0922-4106(89)90023-0. [DOI] [PubMed] [Google Scholar]
- Garthwaite J. Glutamate, nitric oxide and cell-cell signalling in the nervous system. Trends Neurosci. 1991 Feb;14(2):60–67. doi: 10.1016/0166-2236(91)90022-m. [DOI] [PubMed] [Google Scholar]
- Guevara-Guzman R., Emson P. C., Kendrick K. M. Modulation of in vivo striatal transmitter release by nitric oxide and cyclic GMP. J Neurochem. 1994 Feb;62(2):807–810. doi: 10.1046/j.1471-4159.1994.62020807.x. [DOI] [PubMed] [Google Scholar]
- Haberny K. A., Pou S., Eccles C. U. Potentiation of quinolinate-induced hippocampal lesions by inhibition of NO synthesis. Neurosci Lett. 1992 Nov 9;146(2):187–190. doi: 10.1016/0304-3940(92)90074-h. [DOI] [PubMed] [Google Scholar]
- Hanbauer I., Wink D., Osawa Y., Edelman G. M., Gally J. A. Role of nitric oxide in NMDA-evoked release of [3H]-dopamine from striatal slices. Neuroreport. 1992 May;3(5):409–412. doi: 10.1097/00001756-199205000-00008. [DOI] [PubMed] [Google Scholar]
- Hölscher C., Rose S. P. Inhibiting synthesis of the putative retrograde messenger nitric oxide results in amnesia in a passive avoidance task in the chick. Brain Res. 1993 Aug 13;619(1-2):189–194. doi: 10.1016/0006-8993(93)91611-u. [DOI] [PubMed] [Google Scholar]
- Ignarro L. J. Biosynthesis and metabolism of endothelium-derived nitric oxide. Annu Rev Pharmacol Toxicol. 1990;30:535–560. doi: 10.1146/annurev.pa.30.040190.002535. [DOI] [PubMed] [Google Scholar]
- Khanna J. M., Morato G. S., Shah G., Chau A., Kalant H. Inhibition of nitric oxide synthesis impairs rapid tolerance to ethanol. Brain Res Bull. 1993;32(1):43–47. doi: 10.1016/0361-9230(93)90317-5. [DOI] [PubMed] [Google Scholar]
- Knowles R. G., Palacios M., Palmer R. M., Moncada S. Formation of nitric oxide from L-arginine in the central nervous system: a transduction mechanism for stimulation of the soluble guanylate cyclase. Proc Natl Acad Sci U S A. 1989 Jul;86(13):5159–5162. doi: 10.1073/pnas.86.13.5159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komori Y., Chiang K. T., Fukuto J. M. The effect of nonionic detergents on the activity and/or stability of rat brain nitric oxide synthase. Arch Biochem Biophys. 1993 Dec;307(2):311–315. doi: 10.1006/abbi.1993.1594. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lei S. Z., Pan Z. H., Aggarwal S. K., Chen H. S., Hartman J., Sucher N. J., Lipton S. A. Effect of nitric oxide production on the redox modulatory site of the NMDA receptor-channel complex. Neuron. 1992 Jun;8(6):1087–1099. doi: 10.1016/0896-6273(92)90130-6. [DOI] [PubMed] [Google Scholar]
- Lipton S. A., Choi Y. B., Pan Z. H., Lei S. Z., Chen H. S., Sucher N. J., Loscalzo J., Singel D. J., Stamler J. S. A redox-based mechanism for the neuroprotective and neurodestructive effects of nitric oxide and related nitroso-compounds. Nature. 1993 Aug 12;364(6438):626–632. doi: 10.1038/364626a0. [DOI] [PubMed] [Google Scholar]
- Löscher W., Annies R., Hönack D. The N-methyl-D-aspartate receptor antagonist MK-801 induces increases in dopamine and serotonin metabolism in several brain regions of rats. Neurosci Lett. 1991 Jul 22;128(2):191–194. doi: 10.1016/0304-3940(91)90258-u. [DOI] [PubMed] [Google Scholar]
- Manzoni O., Prezeau L., Marin P., Desagher S., Deshager S., Bockaert J., Fagni L. Nitric oxide-induced blockade of NMDA receptors. Neuron. 1992 Apr;8(4):653–662. doi: 10.1016/0896-6273(92)90087-t. [DOI] [PubMed] [Google Scholar]
- Meffert M. K., Premack B. A., Schulman H. Nitric oxide stimulates Ca(2+)-independent synaptic vesicle release. Neuron. 1994 Jun;12(6):1235–1244. doi: 10.1016/0896-6273(94)90440-5. [DOI] [PubMed] [Google Scholar]
- Moncada S., Palmer R. M., Higgs E. A. Biosynthesis of nitric oxide from L-arginine. A pathway for the regulation of cell function and communication. Biochem Pharmacol. 1989 Jun 1;38(11):1709–1715. doi: 10.1016/0006-2952(89)90403-6. [DOI] [PubMed] [Google Scholar]
- Montague P. R., Gancayco C. D., Winn M. J., Marchase R. B., Friedlander M. J. Role of NO production in NMDA receptor-mediated neurotransmitter release in cerebral cortex. Science. 1994 Feb 18;263(5149):973–977. doi: 10.1126/science.7508638. [DOI] [PubMed] [Google Scholar]
- Nabeshima T., Fukaya H., Yamaguchi K., Ishikawa K., Furukawa H., Kameyama T. Development of tolerance and supersensitivity to phencyclidine in rats after repeated administration of phencyclidine. Eur J Pharmacol. 1987 Mar 3;135(1):23–33. doi: 10.1016/0014-2999(87)90753-9. [DOI] [PubMed] [Google Scholar]
- Nitta A., Furukawa Y., Hayashi K., Hiramatsu M., Kameyama T., Hasegawa T., Nabeshima T. Denervation of dopaminergic neurons with 6-hydroxydopamine increases nerve growth factor content in rat brain. Neurosci Lett. 1992 Sep 14;144(1-2):152–156. doi: 10.1016/0304-3940(92)90738-s. [DOI] [PubMed] [Google Scholar]
- Nitta A., Murakami Y., Furukawa Y., Kawatsura W., Hayashi K., Yamada K., Hasegawa T., Nabeshima T. Oral administration of idebenone induces nerve growth factor in the brain and improves learning and memory in basal forebrain-lesioned rats. Naunyn Schmiedebergs Arch Pharmacol. 1994 Apr;349(4):401–407. doi: 10.1007/BF00170887. [DOI] [PubMed] [Google Scholar]
- Nowicki J. P., Duval D., Poignet H., Scatton B. Nitric oxide mediates neuronal death after focal cerebral ischemia in the mouse. Eur J Pharmacol. 1991 Nov 12;204(3):339–340. doi: 10.1016/0014-2999(91)90862-k. [DOI] [PubMed] [Google Scholar]
- O'Dell T. J., Hawkins R. D., Kandel E. R., Arancio O. Tests of the roles of two diffusible substances in long-term potentiation: evidence for nitric oxide as a possible early retrograde messenger. Proc Natl Acad Sci U S A. 1991 Dec 15;88(24):11285–11289. doi: 10.1073/pnas.88.24.11285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platel A., Porsolt R. D. Habituation of exploratory activity in mice: a screening test for memory enhancing drugs. Psychopharmacology (Berl) 1982;78(4):346–352. doi: 10.1007/BF00433739. [DOI] [PubMed] [Google Scholar]
- Pudiak C. M., Bozarth M. A. L-NAME and MK-801 attenuate sensitization to the locomotor-stimulating effect of cocaine. Life Sci. 1993;53(20):1517–1524. doi: 10.1016/0024-3205(93)90559-l. [DOI] [PubMed] [Google Scholar]
- Rauhala P., Idänpän-Heikkilä J. J., Tuominen R. K., Männistö P. T. N-nitro-L-arginine attenuates development of tolerance to antinociceptive but not to hormonal effects of morphine. Eur J Pharmacol. 1994 Jun 23;259(1):57–64. doi: 10.1016/0014-2999(94)90157-0. [DOI] [PubMed] [Google Scholar]
- Rees D. D., Palmer R. M., Schulz R., Hodson H. F., Moncada S. Characterization of three inhibitors of endothelial nitric oxide synthase in vitro and in vivo. Br J Pharmacol. 1990 Nov;101(3):746–752. doi: 10.1111/j.1476-5381.1990.tb14151.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuman E. M., Madison D. V. A requirement for the intercellular messenger nitric oxide in long-term potentiation. Science. 1991 Dec 6;254(5037):1503–1506. doi: 10.1126/science.1720572. [DOI] [PubMed] [Google Scholar]
- Snyder S. H. Nitric oxide: first in a new class of neurotransmitters. Science. 1992 Jul 24;257(5069):494–496. doi: 10.1126/science.1353273. [DOI] [PubMed] [Google Scholar]
- Southam E., Garthwaite J. The nitric oxide-cyclic GMP signalling pathway in rat brain. Neuropharmacology. 1993 Nov;32(11):1267–1277. doi: 10.1016/0028-3908(93)90021-t. [DOI] [PubMed] [Google Scholar]
- Vincent S. R., Kimura H. Histochemical mapping of nitric oxide synthase in the rat brain. Neuroscience. 1992;46(4):755–784. doi: 10.1016/0306-4522(92)90184-4. [DOI] [PubMed] [Google Scholar]
- Ward L., Mason S. E., Abraham W. C. Effects of the NMDA antagonists CPP and MK-801 on radial arm maze performance in rats. Pharmacol Biochem Behav. 1990 Apr;35(4):785–790. doi: 10.1016/0091-3057(90)90359-p. [DOI] [PubMed] [Google Scholar]
- Whitton P. S., Biggs C. S., Pearce B. R., Fowler L. J. MK-801 increases extracellular 5-hydroxytryptamine in rat hippocampus and striatum in vivo. J Neurochem. 1992 Apr;58(4):1573–1575. doi: 10.1111/j.1471-4159.1992.tb11381.x. [DOI] [PubMed] [Google Scholar]
- Zhu X. Z., Luo L. G. Effect of nitroprusside (nitric oxide) on endogenous dopamine release from rat striatal slices. J Neurochem. 1992 Sep;59(3):932–935. doi: 10.1111/j.1471-4159.1992.tb08332.x. [DOI] [PubMed] [Google Scholar]
- Zhuo M., Hu Y., Schultz C., Kandel E. R., Hawkins R. D. Role of guanylyl cyclase and cGMP-dependent protein kinase in long-term potentiation. Nature. 1994 Apr 14;368(6472):635–639. doi: 10.1038/368635a0. [DOI] [PubMed] [Google Scholar]