Abstract
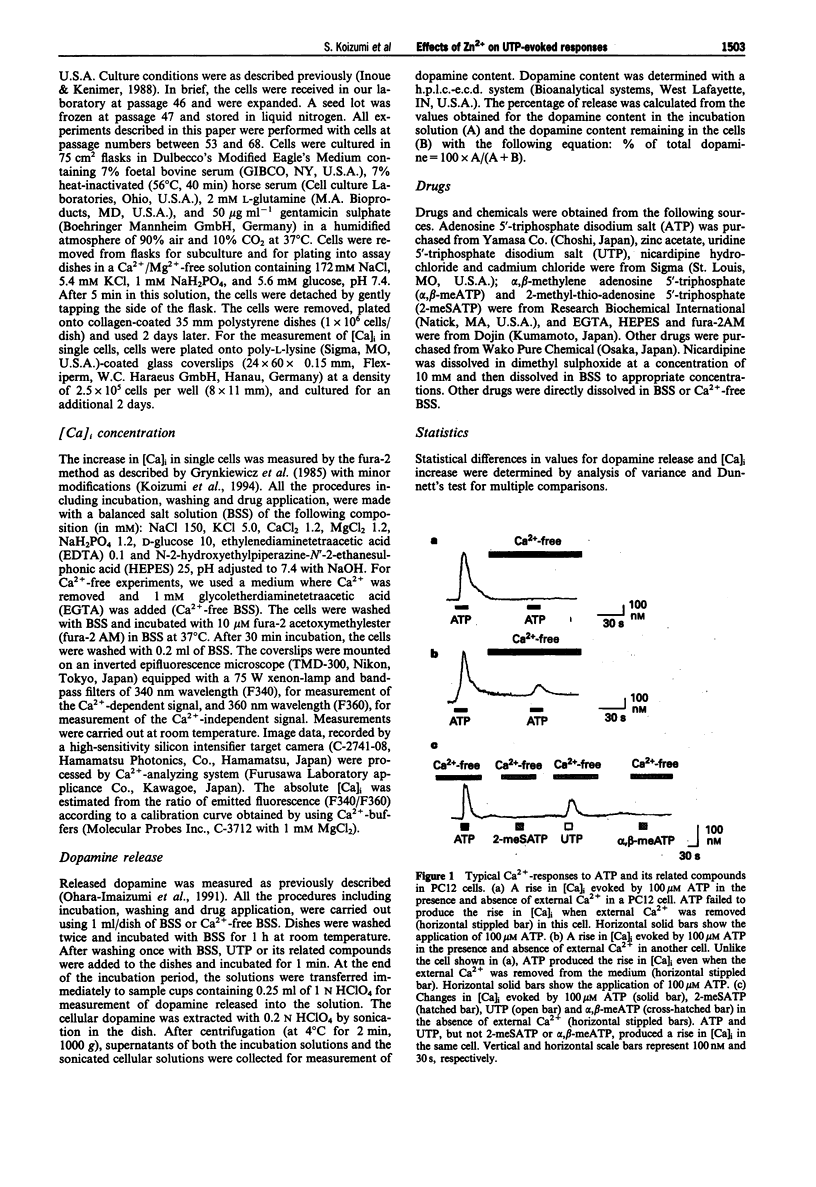
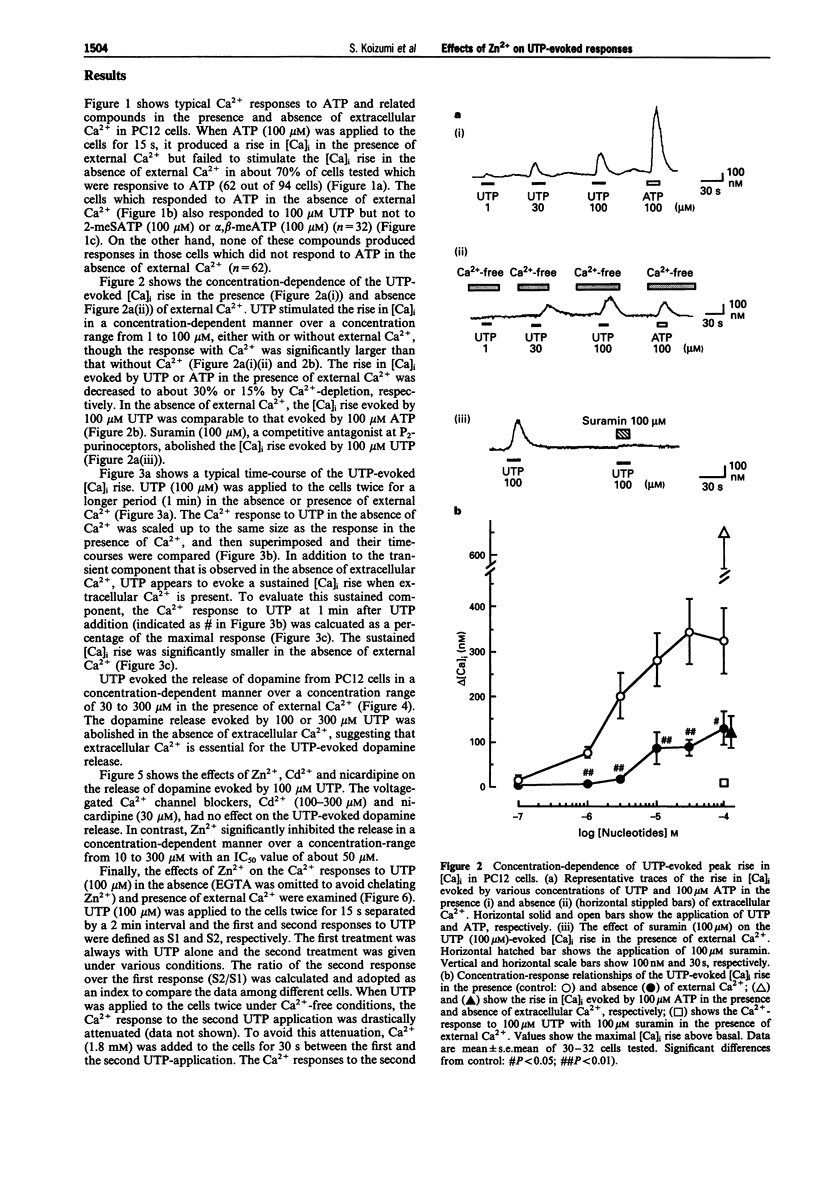
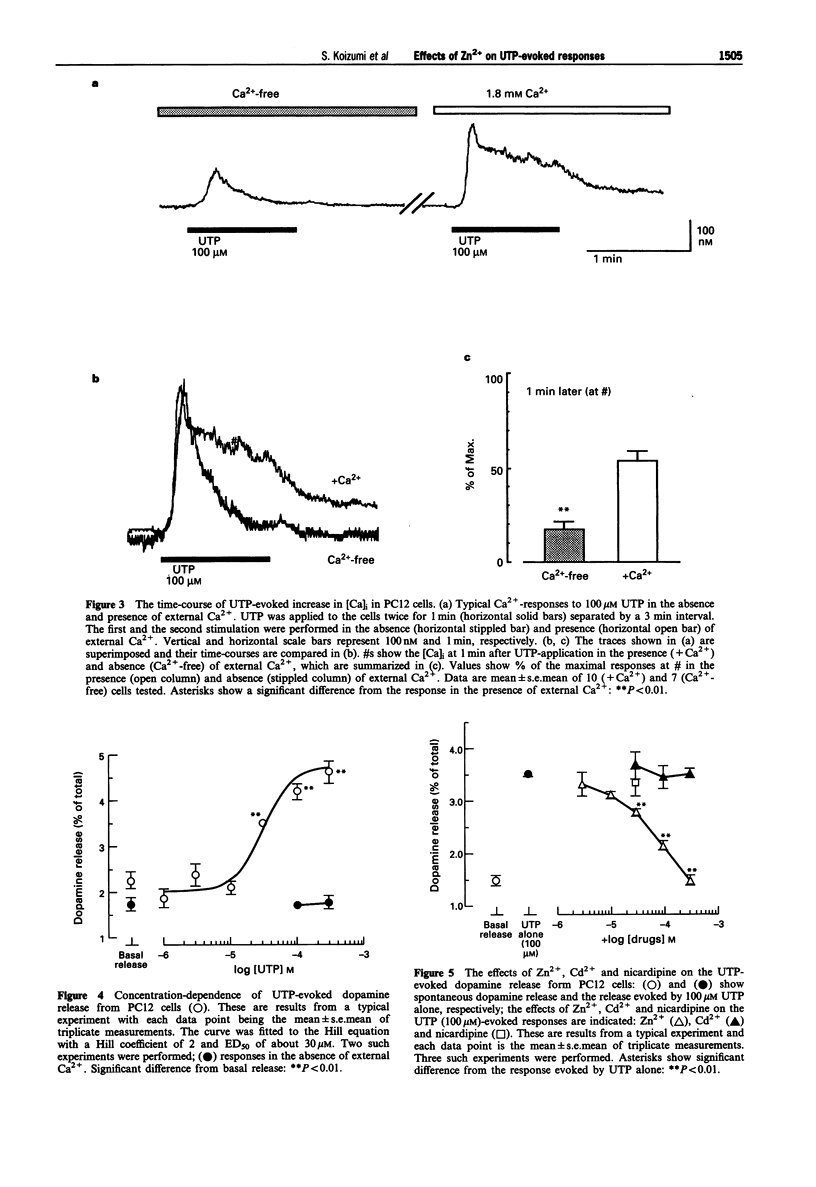
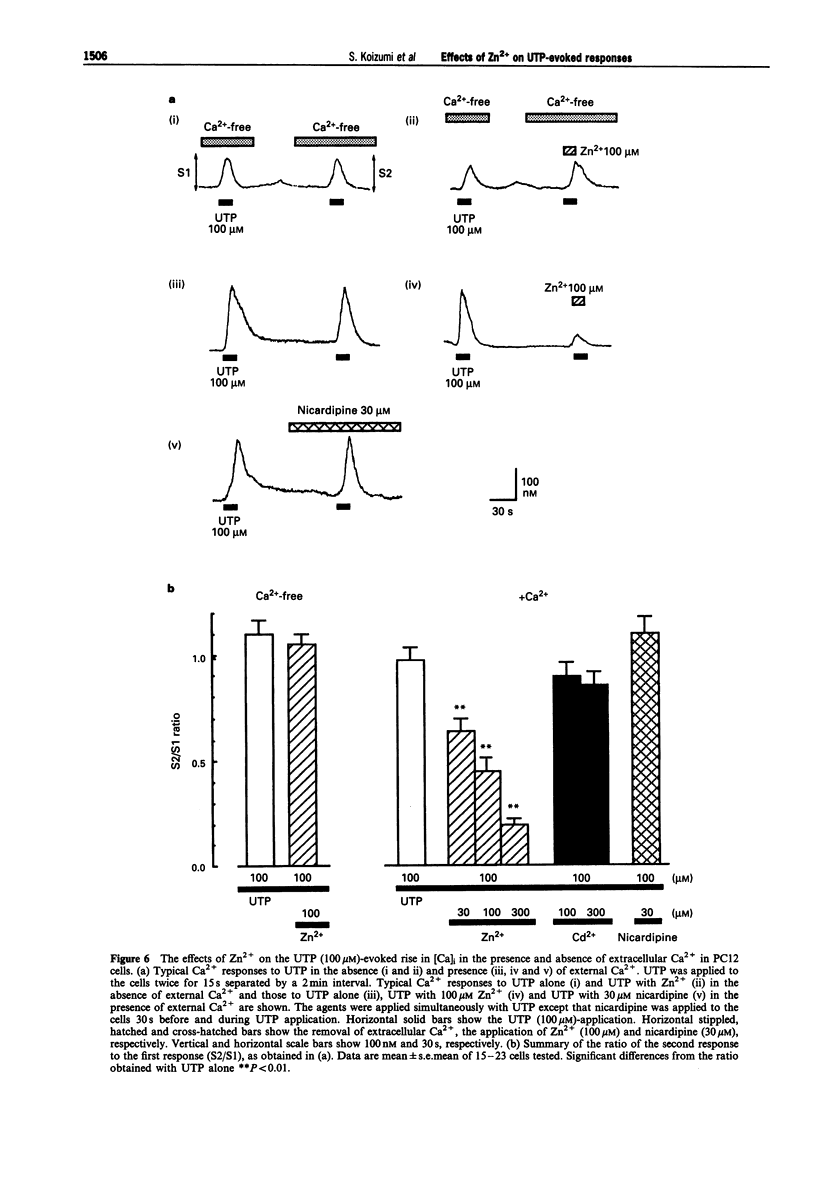
1. Uridine 5'-triphosphate (UTP)-evoked increase in intracellular Ca2+ concentration ([Ca]i) and release of dopamine were investigated in rat phaeochromocytoma PC12 cells. UTP (1-100 microM) evoked an increase in [Ca]i in a concentration-dependent manner. This response was decreased to about 30% by extracellular Ca(2+)-depletion, but not abolished. This [Ca]i rise was mimicked by 100 microM ATP but not by 100 microM 2-methyl-thio-ATP or alpha,beta-methylene-ATP in the absence of external Ca2+, suggesting that the response was mediated by P2U purinoceptors, a subclass of P2-purinoceptors. 2. The UTP-evoked [Ca]i rise consisted of two components; a transient and a sustained one. When external Ca2+ was removed, the sustained component was abolished while the transient component was decreased by about 70% but did not disappear. These results suggest that UTP induces Ca(2+)-mobilization and, subsequently, Ca(2+)-influx. 3. The UTP-evoked increase in [Ca]i was not affected by Cd2+ (100 and 300 microM) or nicardipine (30 microM), inhibitors of voltage-gated calcium channels, but was significantly inhibited by Zn2+ (10-300 microM) in the presence of external Ca2+. Zn2+, however, did not affect the Ca2+ response to UTP in the absence of external Ca2+. 4. UTP (30 microM-1 mM) evoked the release of dopamine from the cells in a concentration-dependent manner. This dopamine release was abolished by Ca(2+)-depletion or Zn2+ but not by Cd2+ or nicardipine. 5. Taken together, the data demonstrate that UTP stimulates P2U-purinoceptors and induces a rise in [Ca]i both by Ca(2+)-mobilization and Ca(2+)-influx in PC12 cells.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abbracchio M. P., Burnstock G. Purinoceptors: are there families of P2X and P2Y purinoceptors? Pharmacol Ther. 1994;64(3):445–475. doi: 10.1016/0163-7258(94)00048-4. [DOI] [PubMed] [Google Scholar]
- Barry V. A., Cheek T. R. Extracellular ATP triggers two functionally distinct calcium signalling pathways in PC12 cells. J Cell Sci. 1994 Feb;107(Pt 2):451–462. doi: 10.1242/jcs.107.2.451. [DOI] [PubMed] [Google Scholar]
- Bean B. P. Pharmacology and electrophysiology of ATP-activated ion channels. Trends Pharmacol Sci. 1992 Mar;13(3):87–90. doi: 10.1016/0165-6147(92)90032-2. [DOI] [PubMed] [Google Scholar]
- Brake A. J., Wagenbach M. J., Julius D. New structural motif for ligand-gated ion channels defined by an ionotropic ATP receptor. Nature. 1994 Oct 6;371(6497):519–523. doi: 10.1038/371519a0. [DOI] [PubMed] [Google Scholar]
- Burnstock G., Kennedy C. Is there a basis for distinguishing two types of P2-purinoceptor? Gen Pharmacol. 1985;16(5):433–440. doi: 10.1016/0306-3623(85)90001-1. [DOI] [PubMed] [Google Scholar]
- Clementi E., Scheer H., Zacchetti D., Fasolato C., Pozzan T., Meldolesi J. Receptor-activated Ca2+ influx. Two independently regulated mechanisms of influx stimulation coexist in neurosecretory PC12 cells. J Biol Chem. 1992 Feb 5;267(4):2164–2172. [PubMed] [Google Scholar]
- Cloues R., Jones S., Brown D. A. Zn2+ potentiates ATP-activated currents in rat sympathetic neurons. Pflugers Arch. 1993 Jul;424(2):152–158. doi: 10.1007/BF00374606. [DOI] [PubMed] [Google Scholar]
- Edwards F. A., Gibb A. J., Colquhoun D. ATP receptor-mediated synaptic currents in the central nervous system. Nature. 1992 Sep 10;359(6391):144–147. doi: 10.1038/359144a0. [DOI] [PubMed] [Google Scholar]
- Evans R. J., Derkach V., Surprenant A. ATP mediates fast synaptic transmission in mammalian neurons. Nature. 1992 Jun 11;357(6378):503–505. doi: 10.1038/357503a0. [DOI] [PubMed] [Google Scholar]
- Frederickson C. J. Neurobiology of zinc and zinc-containing neurons. Int Rev Neurobiol. 1989;31:145–238. doi: 10.1016/s0074-7742(08)60279-2. [DOI] [PubMed] [Google Scholar]
- Fredholm B. B., Abbracchio M. P., Burnstock G., Daly J. W., Harden T. K., Jacobson K. A., Leff P., Williams M. Nomenclature and classification of purinoceptors. Pharmacol Rev. 1994 Jun;46(2):143–156. [PMC free article] [PubMed] [Google Scholar]
- Greene L. A., Tischler A. S. Establishment of a noradrenergic clonal line of rat adrenal pheochromocytoma cells which respond to nerve growth factor. Proc Natl Acad Sci U S A. 1976 Jul;73(7):2424–2428. doi: 10.1073/pnas.73.7.2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- Harrison N. L., Gibbons S. J. Zn2+: an endogenous modulator of ligand- and voltage-gated ion channels. Neuropharmacology. 1994 Aug;33(8):935–952. doi: 10.1016/0028-3908(94)90152-x. [DOI] [PubMed] [Google Scholar]
- Inoue K., Kenimer J. G. Muscarinic stimulation of calcium influx and norepinephrine release in PC12 cells. J Biol Chem. 1988 Jun 15;263(17):8157–8161. [PubMed] [Google Scholar]
- Inoue K., Koizumi S., Nakazawa K. Glutamate-evoked release of adenosine 5'-triphosphate causing an increase in intracellular calcium in hippocampal neurons. Neuroreport. 1995 Feb 15;6(3):437–440. doi: 10.1097/00001756-199502000-00008. [DOI] [PubMed] [Google Scholar]
- Inoue K., Nakazawa K., Fujimori K., Takanaka A. Extracellular adenosine 5'-triphosphate-evoked norepinephrine secretion not relating to voltage-gated Ca channels in pheochromocytoma PC12 cells. Neurosci Lett. 1989 Dec 4;106(3):294–299. doi: 10.1016/0304-3940(89)90179-1. [DOI] [PubMed] [Google Scholar]
- Inoue K., Nakazawa K., Fujimori K., Watano T., Takanaka A. Extracellular adenosine 5'-triphosphate-evoked glutamate release in cultured hippocampal neurons. Neurosci Lett. 1992 Jan 6;134(2):215–218. doi: 10.1016/0304-3940(92)90520-h. [DOI] [PubMed] [Google Scholar]
- Koizumi S., Ikeda M., Inoue K., Nakazawa K., Inoue K. Enhancement by zinc of ATP-evoked dopamine release from rat pheochromocytoma PC12 cells. Brain Res. 1995 Feb 27;673(1):75–82. doi: 10.1016/0006-8993(94)01404-6. [DOI] [PubMed] [Google Scholar]
- Koizumi S., Watano T., Nakazawa K., Inoue K. Potentiation by adenosine of ATP-evoked dopamine release via a pertussis toxin-sensitive mechanism in rat phaeochromocytoma PC12 cells. Br J Pharmacol. 1994 Jul;112(3):992–997. doi: 10.1111/j.1476-5381.1994.tb13179.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C., Peoples R. W., Li Z., Weight F. F. Zn2+ potentiates excitatory action of ATP on mammalian neurons. Proc Natl Acad Sci U S A. 1993 Sep 1;90(17):8264–8267. doi: 10.1073/pnas.90.17.8264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin T. A., Lustig K. D., Sportiello M. G., Weisman G. A., Sun G. Y. Signal transduction pathways coupled to a P2U receptor in neuroblastoma x glioma (NG108-15) cells. J Neurochem. 1993 Mar;60(3):1115–1125. doi: 10.1111/j.1471-4159.1993.tb03262.x. [DOI] [PubMed] [Google Scholar]
- Lustig K. D., Shiau A. K., Brake A. J., Julius D. Expression cloning of an ATP receptor from mouse neuroblastoma cells. Proc Natl Acad Sci U S A. 1993 Jun 1;90(11):5113–5117. doi: 10.1073/pnas.90.11.5113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majid M. A., Okajima F., Kondo Y. UTP activates phospholipase C-Ca2+ system through a receptor different from the 53-kDa ATP receptor in PC12 cells. Biochem Biophys Res Commun. 1993 Aug 31;195(1):415–421. doi: 10.1006/bbrc.1993.2059. [DOI] [PubMed] [Google Scholar]
- Munshi R., DeBernardi M. A., Brooker G. P2U-purinergic receptors on C6-2B rat glioma cells: modulation of cytosolic Ca2+ and cAMP levels by protein kinase C. Mol Pharmacol. 1993 Dec;44(6):1185–1191. [PubMed] [Google Scholar]
- Murrin R. J., Boarder M. R. Neuronal "nucleotide" receptor linked to phospholipase C and phospholipase D? Stimulation of PC12 cells by ATP analogues and UTP. Mol Pharmacol. 1992 Mar;41(3):561–568. [PubMed] [Google Scholar]
- Nakazawa K., Inoue K. Roles of Ca2+ influx through ATP-activated channels in catecholamine release from pheochromocytoma PC12 cells. J Neurophysiol. 1992 Dec;68(6):2026–2032. doi: 10.1152/jn.1992.68.6.2026. [DOI] [PubMed] [Google Scholar]
- Nikodijevic B., Sei Y., Shin Y., Daly J. W. Effects of ATP and UTP in pheochromocytoma PC12 cells: evidence for the presence of three P2 receptors, only one of which subserves stimulation of norepinephrine release. Cell Mol Neurobiol. 1994 Feb;14(1):27–47. doi: 10.1007/BF02088587. [DOI] [PubMed] [Google Scholar]
- Ohara-Imaizumi M., Nakazawa K., Obama T., Fujimori K., Takanaka A., Inoue K. Inhibitory action of peripheral-type benzodiazepines on dopamine release from PC12 pheochromocytoma cells. J Pharmacol Exp Ther. 1991 Nov;259(2):484–489. [PubMed] [Google Scholar]
- Pacaud P., Loirand G., Grégoire G., Mironneau C., Mironneau J. Noradrenaline-activated heparin-sensitive Ca2+ entry after depletion of intracellular Ca2+ store in portal vein smooth muscle cells. J Biol Chem. 1993 Feb 25;268(6):3866–3872. [PubMed] [Google Scholar]
- Putney J. W., Jr A model for receptor-regulated calcium entry. Cell Calcium. 1986 Feb;7(1):1–12. doi: 10.1016/0143-4160(86)90026-6. [DOI] [PubMed] [Google Scholar]
- Raha S., de Souza L. R., Reed J. K. Intracellular signalling by nucleotide receptors in PC12 pheochromocytoma cells. J Cell Physiol. 1993 Mar;154(3):623–630. doi: 10.1002/jcp.1041540322. [DOI] [PubMed] [Google Scholar]
- Randriamampita C., Tsien R. Y. Emptying of intracellular Ca2+ stores releases a novel small messenger that stimulates Ca2+ influx. Nature. 1993 Aug 26;364(6440):809–814. doi: 10.1038/364809a0. [DOI] [PubMed] [Google Scholar]
- Rassendren F. A., Lory P., Pin J. P., Nargeot J. Zinc has opposite effects on NMDA and non-NMDA receptors expressed in Xenopus oocytes. Neuron. 1990 May;4(5):733–740. doi: 10.1016/0896-6273(90)90199-p. [DOI] [PubMed] [Google Scholar]
- Valera S., Hussy N., Evans R. J., Adami N., North R. A., Surprenant A., Buell G. A new class of ligand-gated ion channel defined by P2x receptor for extracellular ATP. Nature. 1994 Oct 6;371(6497):516–519. doi: 10.1038/371516a0. [DOI] [PubMed] [Google Scholar]
- Webb T. E., Simon J., Krishek B. J., Bateson A. N., Smart T. G., King B. F., Burnstock G., Barnard E. A. Cloning and functional expression of a brain G-protein-coupled ATP receptor. FEBS Lett. 1993 Jun 14;324(2):219–225. doi: 10.1016/0014-5793(93)81397-i. [DOI] [PubMed] [Google Scholar]


