Abstract
1. Our previous study demonstrated that YC-1, a derivative of benzylindazole, is a novel activator of soluble guanylate cyclase (sGC) in rabbit platelets. This work investigated whether the antiplatelet effect of YC-1 was mediated by a nitric oxide (NO)/sGC/cyclic GMP pathway in human platelets. 2. In human washed platelets, YC-1 inhibited platelet aggregation and ATP released induced by U46619 (2 microM), collagen (10 micro ml(-1)) and thrombin (0.1 u ml(-1)) in a concentration-dependent manner with IC50 values of (microM) 2.1 +/- 0.03, 11.7 +/- 2.1 and 59.3 +/- 7.1, respectively. 3. In a 30,000 g supernatant fraction from human platelet homogenate, YC-1 (5-100 microM) increased sGC activity in a concentration-dependent manner. At the same concentration-range, YC-1 elevated cyclic GMP levels markedly, but only slightly elevated cyclic AMP levels in the intact platelets. 4. MY-5445, a selective inhibitor of cyclic GMP phosphodiesterase, potentiated the increases in cyclic GMP caused by YC-1, and shifted the concentration-anti-aggregation curve of YC-1 to the left. In contrast, HL-725, a selective inhibitor of cyclic AMP phosphodiesterase, did not affect either the increases in cyclic nucleotides or the anti-aggregatory effect caused by YC-1. 5. Methylene blue, an inhibitor of sGC, blocked the increases of cyclic GMP caused by YC-1, and attenuated markedly the anti-aggregatory effect of YC-1. The adenylate cyclase inhibitor, 2',5'-dideoxyadenosine (DDA) did not affect YC-1-induced inhibition of platelet aggregation. 6. Haemoglobin, which binds NO, prevented the activation of sGC and anti-aggregatory effect caused by sodium nitroprusside, but did not affect YC-1 response. 7. These results would suggest that YC-1 activates sGC of human platelets by a NO-dependent mechanism, and exerts its antiplatelet effects through the sGC/cyclic GMP pathway.
Full text
PDF
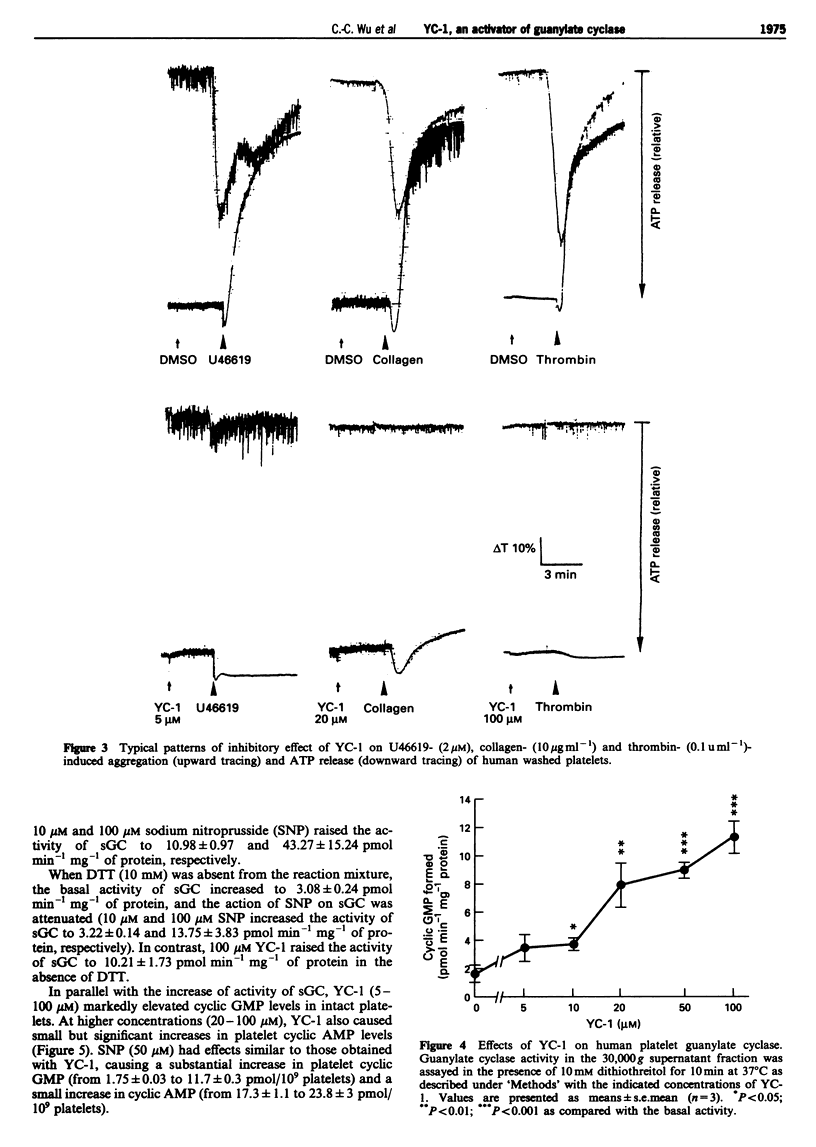
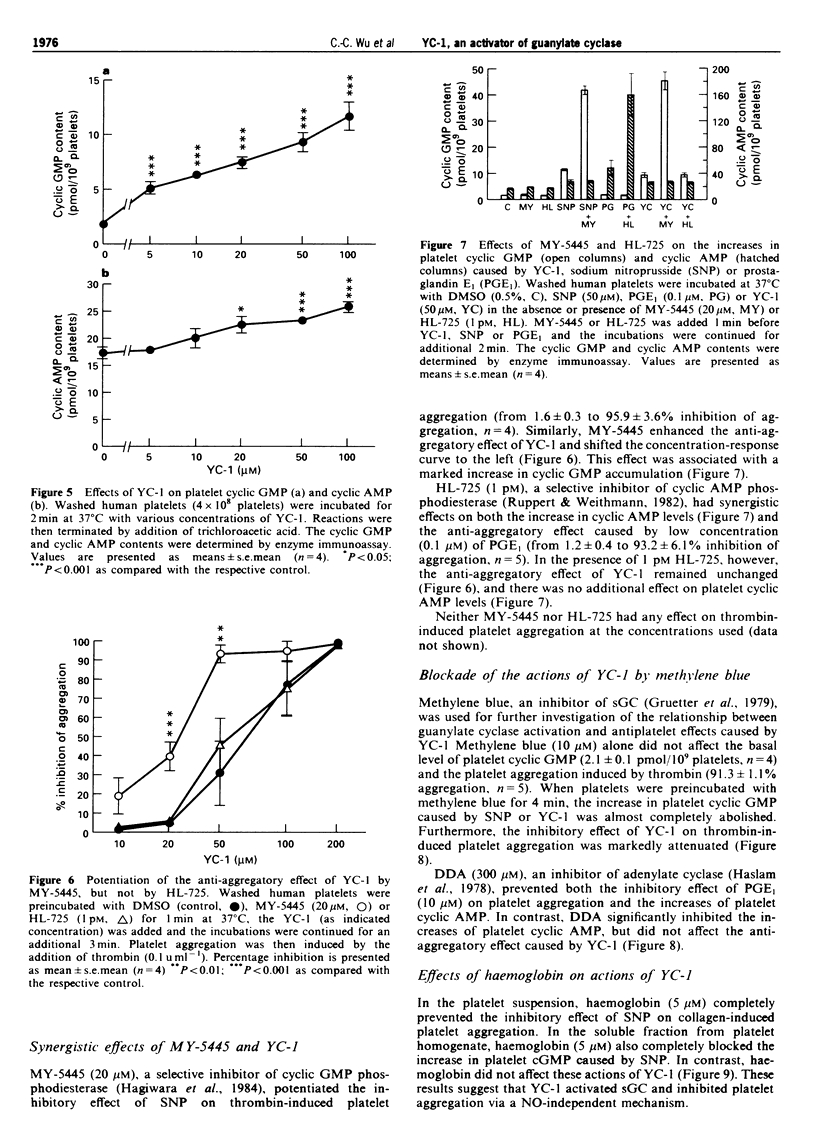
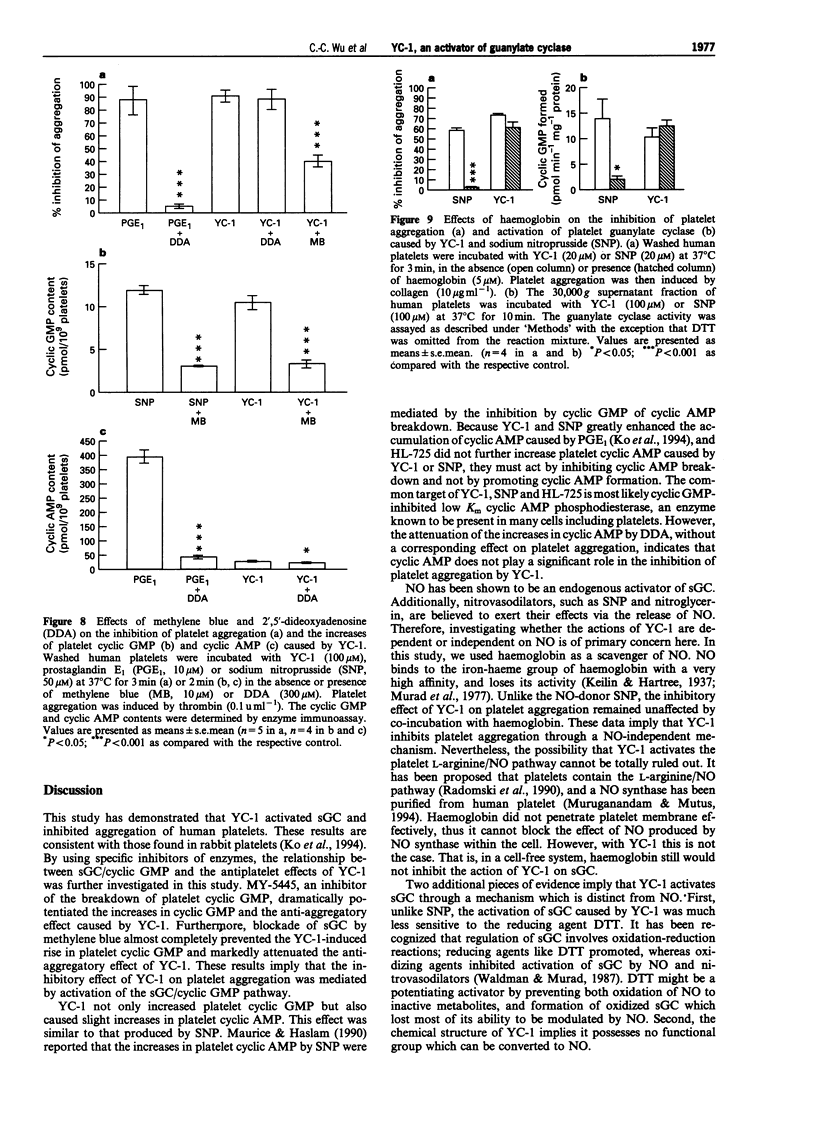

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Buechler W. A., Ivanova K., Wolfram G., Drummer C., Heim J. M., Gerzer R. Soluble guanylyl cyclase and platelet function. Ann N Y Acad Sci. 1994 Apr 18;714:151–157. doi: 10.1111/j.1749-6632.1994.tb12039.x. [DOI] [PubMed] [Google Scholar]
- Gerzer R., Hamet P., Ross A. H., Lawson J. A., Hardman J. G. Calcium-induced release from platelet membranes of fatty acids that modulate soluble guanylate cyclase. J Pharmacol Exp Ther. 1983 Jul;226(1):180–186. [PubMed] [Google Scholar]
- Gruetter C. A., Barry B. K., McNamara D. B., Gruetter D. Y., Kadowitz P. J., Ignarro L. Relaxation of bovine coronary artery and activation of coronary arterial guanylate cyclase by nitric oxide, nitroprusside and a carcinogenic nitrosoamine. J Cyclic Nucleotide Res. 1979;5(3):211–224. [PubMed] [Google Scholar]
- Hagiwara M., Endo T., Kanayama T., Hidaka H. Effect of 1-(3-chloroanilino)-4-phenylphthalazine (MY-5445), a specific inhibitor of cyclic GMP phosphodiesterase, on human platelet aggregation. J Pharmacol Exp Ther. 1984 Feb;228(2):467–471. [PubMed] [Google Scholar]
- Haslam R. J., Davidson M. M., Desjardins J. V. Inhibition of adenylate cyclase by adenosine analogues in preparations of broken and intact human platelets. Evidence for the unidirectional control of platelet function by cyclic AMP. Biochem J. 1978 Oct 15;176(1):83–95. doi: 10.1042/bj1760083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang T. F., Sheu J. R., Teng C. M. A potent antiplatelet peptide, triflavin, from Trimeresurus flavoviridis snake venom. Biochem J. 1991 Jul 15;277(Pt 2):351–357. doi: 10.1042/bj2770351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ignarro L. J., Wood K. S., Wolin M. S. Regulation of purified soluble guanylate cyclase by porphyrins and metalloporphyrins: a unifying concept. Adv Cyclic Nucleotide Protein Phosphorylation Res. 1984;17:267–274. [PubMed] [Google Scholar]
- Ko F. N., Wu C. C., Kuo S. C., Lee F. Y., Teng C. M. YC-1, a novel activator of platelet guanylate cyclase. Blood. 1994 Dec 15;84(12):4226–4233. [PubMed] [Google Scholar]
- Maurice D. H., Haslam R. J. Molecular basis of the synergistic inhibition of platelet function by nitrovasodilators and activators of adenylate cyclase: inhibition of cyclic AMP breakdown by cyclic GMP. Mol Pharmacol. 1990 May;37(5):671–681. [PubMed] [Google Scholar]
- Murad F., Mittal C. K., Arnold W. P., Katsuki S., Kimura H. Guanylate cyclase: activation by azide, nitro compounds, nitric oxide, and hydroxyl radical and inhibition by hemoglobin and myoglobin. Adv Cyclic Nucleotide Res. 1978;9:145–158. [PubMed] [Google Scholar]
- Muruganandam A., Mutus B. Isolation of nitric oxide synthase from human platelets. Biochim Biophys Acta. 1994 May 25;1200(1):1–6. doi: 10.1016/0304-4165(94)90019-1. [DOI] [PubMed] [Google Scholar]
- O'brien J. R. Platelet aggregation: Part I Some effects of the adenosine phosphates, thrombin, and cocaine upon platelet adhesiveness. J Clin Pathol. 1962 Sep;15(5):446–452. doi: 10.1136/jcp.15.5.446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radomski M. W., Palmer R. M., Moncada S. An L-arginine/nitric oxide pathway present in human platelets regulates aggregation. Proc Natl Acad Sci U S A. 1990 Jul;87(13):5193–5197. doi: 10.1073/pnas.87.13.5193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radomski M. W., Palmer R. M., Moncada S. The anti-aggregating properties of vascular endothelium: interactions between prostacyclin and nitric oxide. Br J Pharmacol. 1987 Nov;92(3):639–646. doi: 10.1111/j.1476-5381.1987.tb11367.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruppert D., Weithmann K. U. HL 725, an extremely potent inhibitor of platelet phosphodiesterase and induced platelet aggregation in vitro. Life Sci. 1982 Nov 8;31(19):2037–2043. doi: 10.1016/0024-3205(82)90095-9. [DOI] [PubMed] [Google Scholar]
- Schmidt H. H., Lohmann S. M., Walter U. The nitric oxide and cGMP signal transduction system: regulation and mechanism of action. Biochim Biophys Acta. 1993 Aug 18;1178(2):153–175. doi: 10.1016/0167-4889(93)90006-b. [DOI] [PubMed] [Google Scholar]
- Schmidt H. H. NO., CO and .OH. Endogenous soluble guanylyl cyclase-activating factors. FEBS Lett. 1992 Jul 27;307(1):102–107. doi: 10.1016/0014-5793(92)80910-9. [DOI] [PubMed] [Google Scholar]
- Schmidt H. H., Walter U. NO at work. Cell. 1994 Sep 23;78(6):919–925. doi: 10.1016/0092-8674(94)90267-4. [DOI] [PubMed] [Google Scholar]
- Traylor T. G., Sharma V. S. Why NO? Biochemistry. 1992 Mar 24;31(11):2847–2849. doi: 10.1021/bi00126a001. [DOI] [PubMed] [Google Scholar]
- Waldman S. A., Murad F. Cyclic GMP synthesis and function. Pharmacol Rev. 1987 Sep;39(3):163–196. [PubMed] [Google Scholar]
- Walter U. Physiological role of cGMP and cGMP-dependent protein kinase in the cardiovascular system. Rev Physiol Biochem Pharmacol. 1989;113:41–88. doi: 10.1007/BFb0032675. [DOI] [PubMed] [Google Scholar]
- Yoshina S., Tanaka A., Kuo S. C. [Studies on heterocyclic compounds. XXXIV. Synthesis of furo[3,2-c]pyrazole derivatives. (2). Electrophilic substitution of 1,3-diphenylfuro[3,2-c]pyrazole (author's transl)]. Yakugaku Zasshi. 1978 Feb;98(2):204–209. doi: 10.1248/yakushi1947.98.2_204. [DOI] [PubMed] [Google Scholar]


