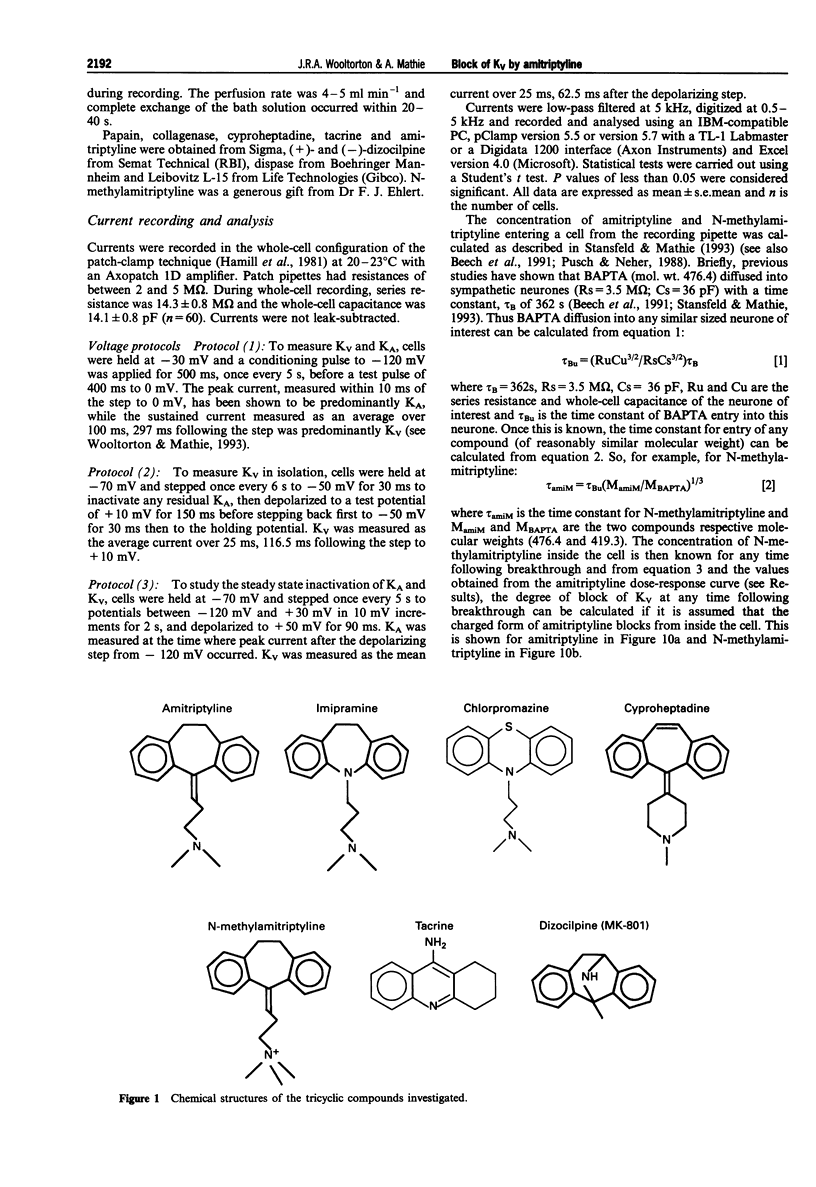
Abstract
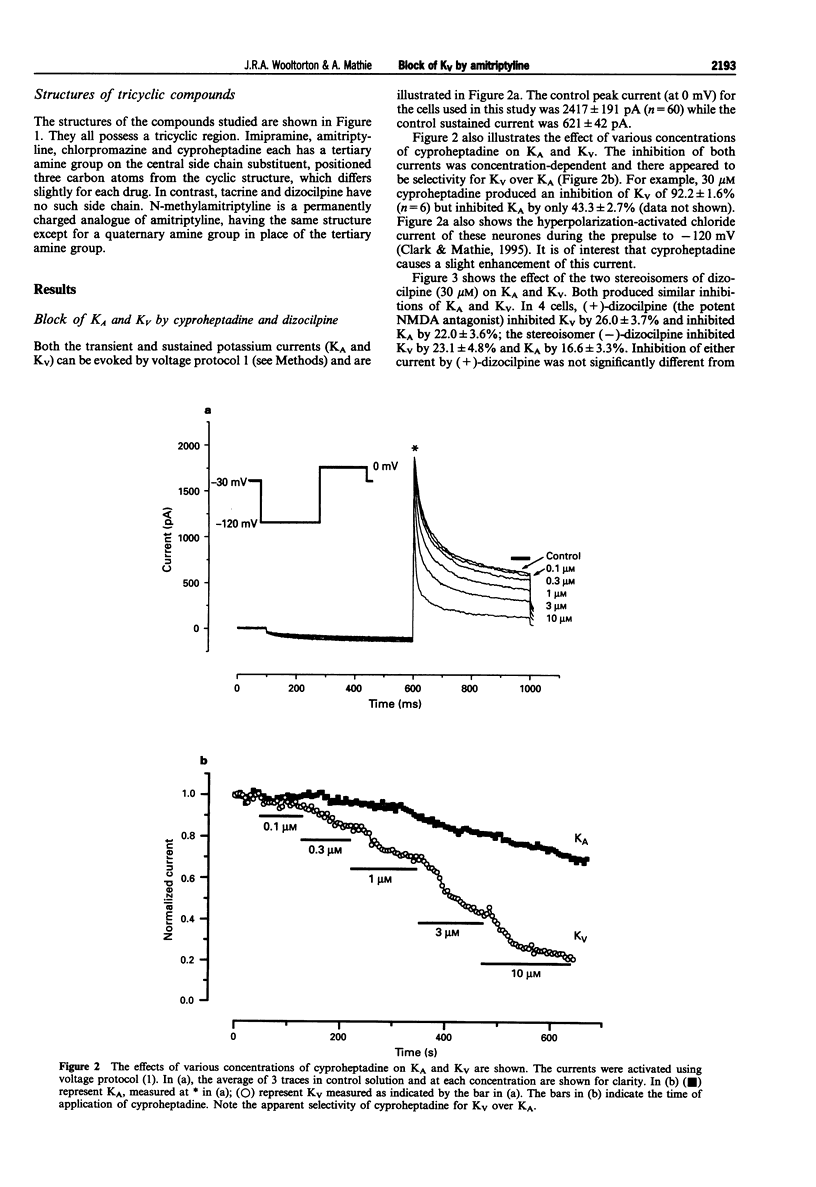
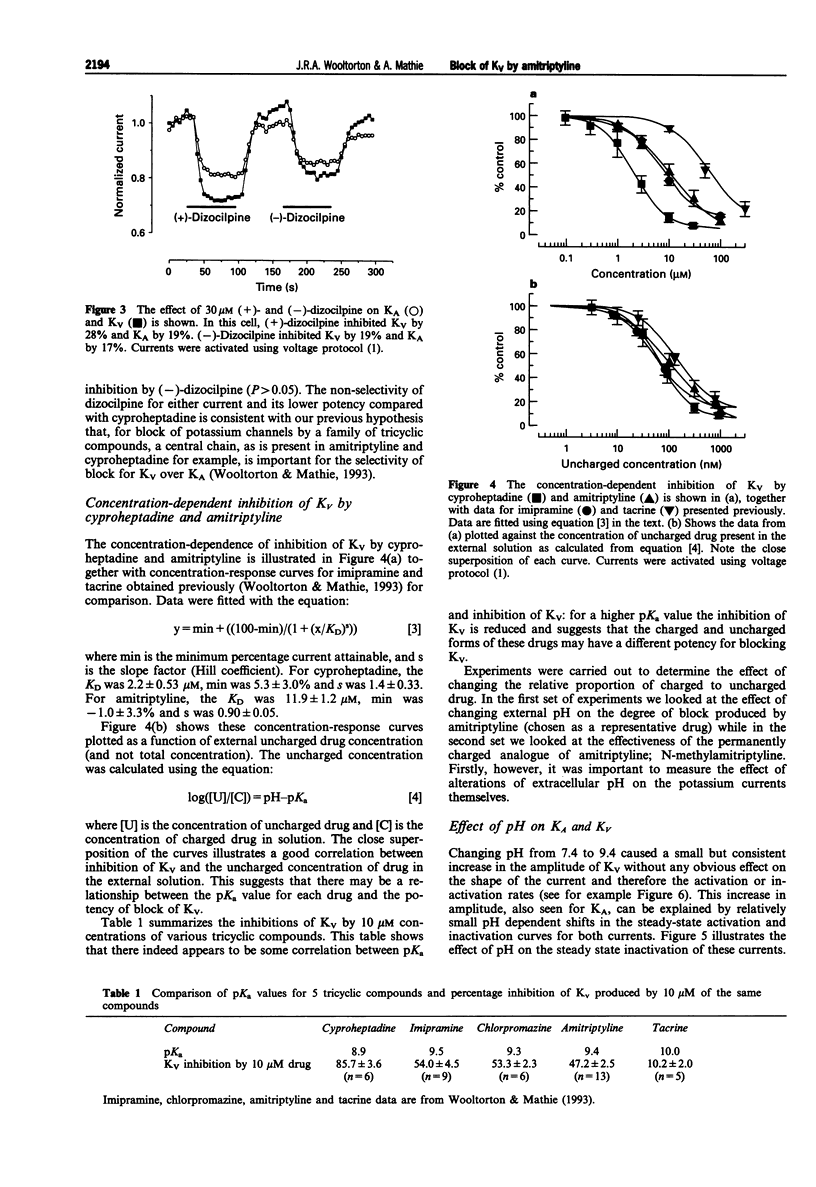
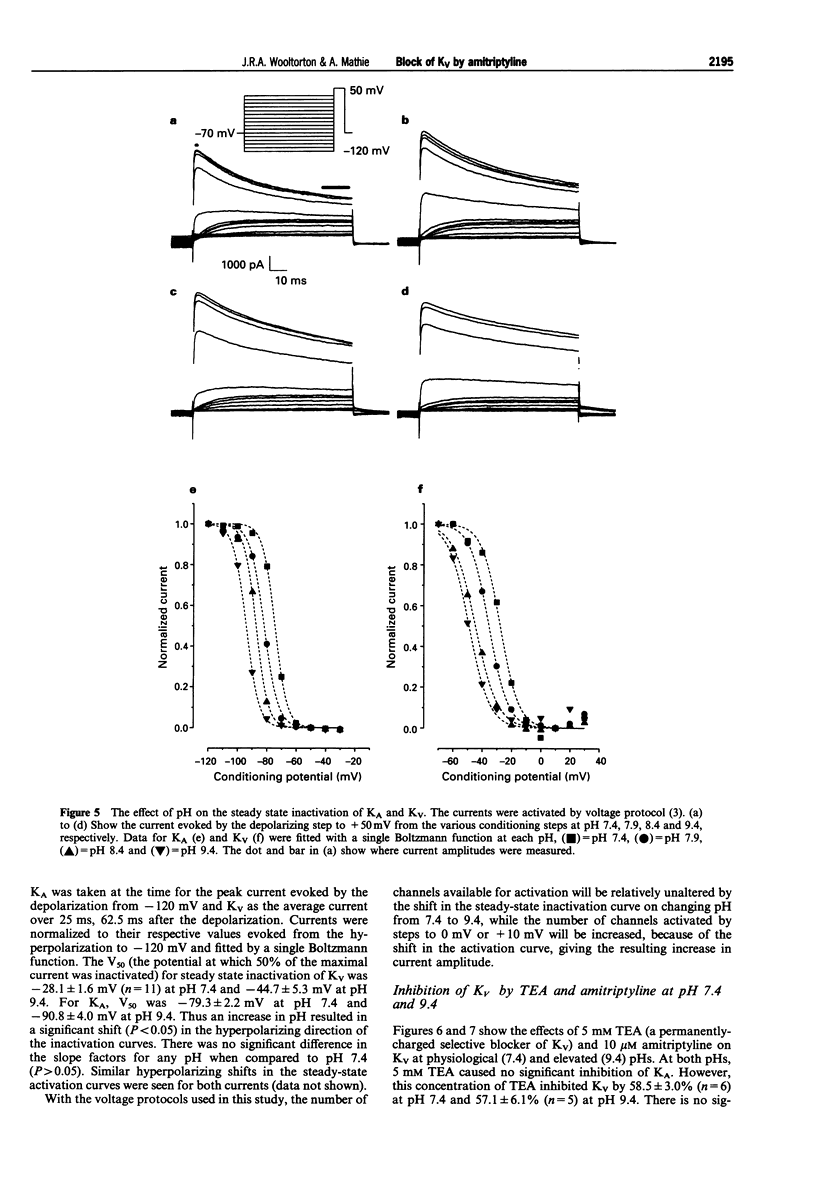
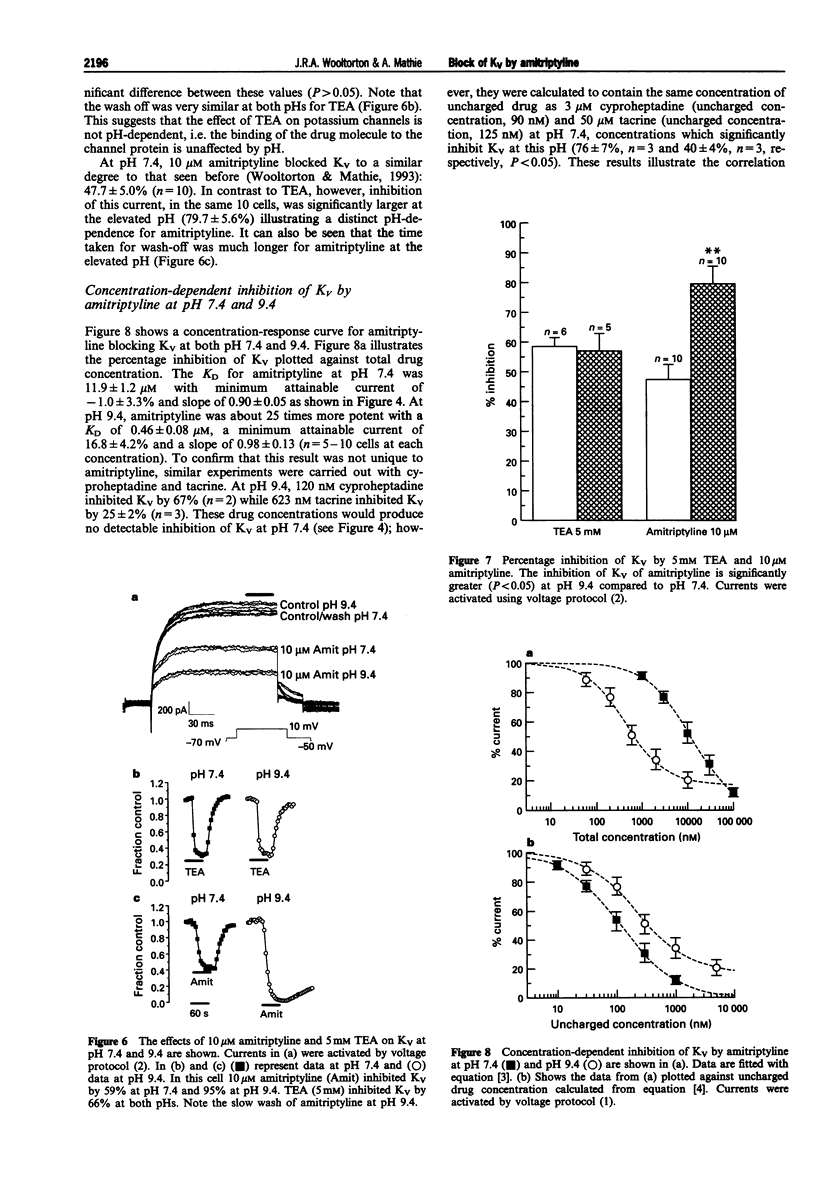
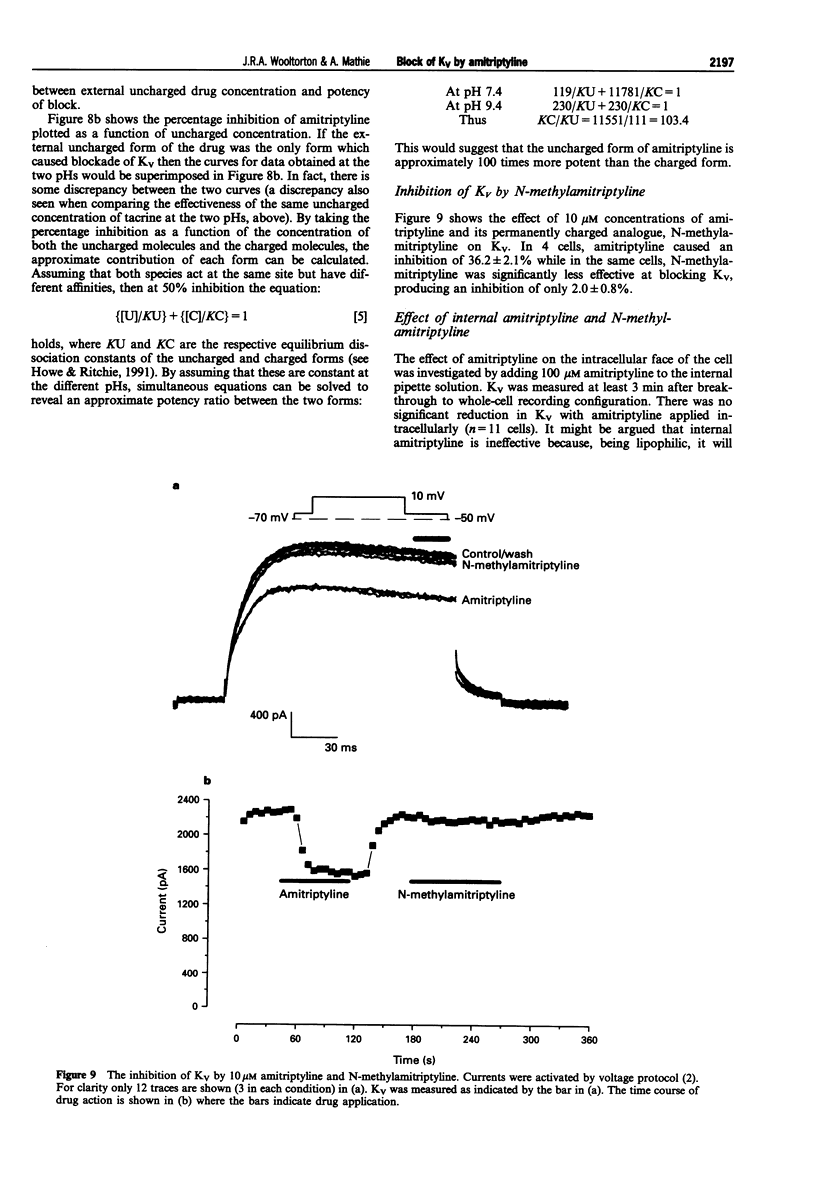
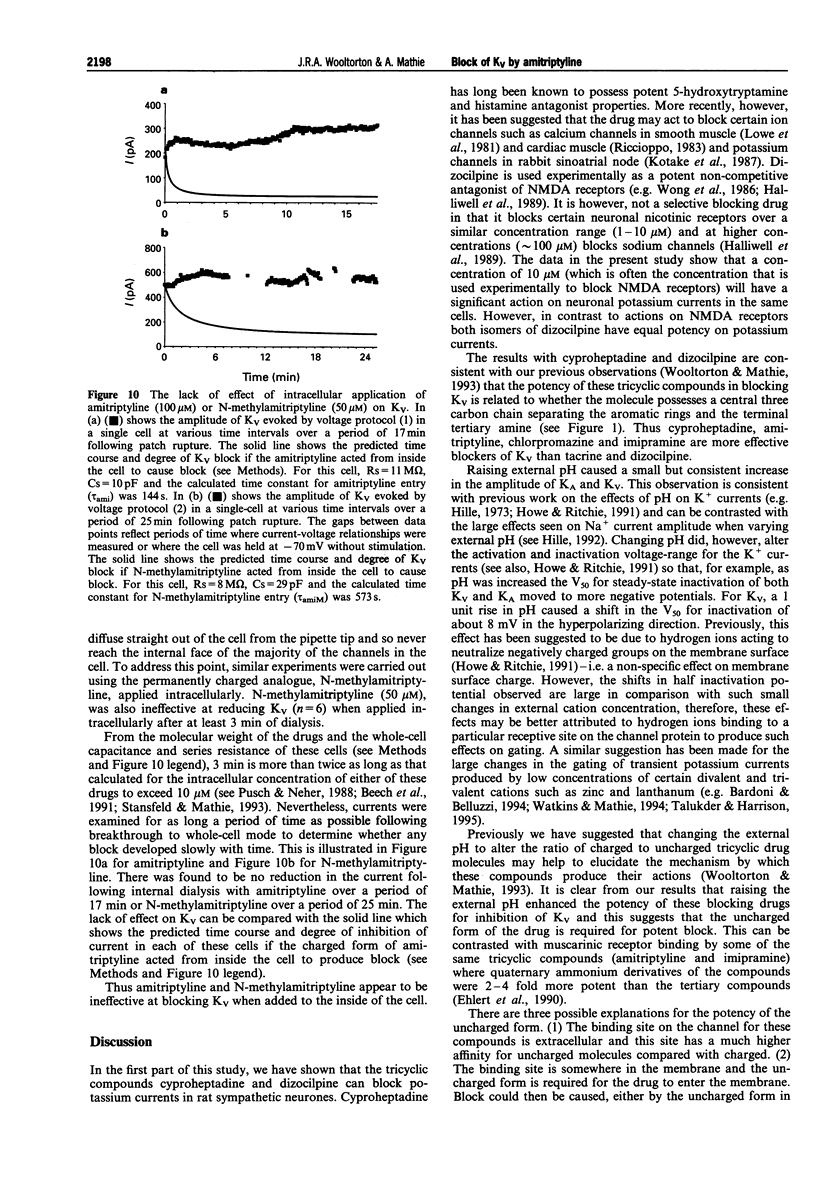
1. The block of K+ currents by amitriptyline and the related tricyclic compounds cyproheptadine and dizocilpine was studied in dissociated rat sympathetic neurones by whole-cell voltage-clamp recording. 2. Cyproheptadine (30 microM) inhibited the delayed-rectifier current (Kv) by 92% and the transient current (KA) by 43%. For inhibition of Kv, cyproheptaidine had a KD of 2.2 microM. Dizocilpine (30 microM) inhibited Kv by 26% and KA by 22%. The stereoisomers of dizocilpine were equally potent at blocking Kv and KA. 3. Amitriptyline, a weak base, was significantly more effective in blocking Kv at pH 9.4 (KD = 0.46 microM) where the ratio of charged to uncharged drug was 50:50 compared with pH 7.4 (KD = 11.9 microM) where the ratio was 99:1. 4. N-methylamitriptyline (10 microM), the permanently charged analogue of amitriptyline, inhibited Kv by only 2% whereas in the same cells amitriptyline (10 microM) inhibited Kv by 36%. 5. Neither amitriptyline nor N-methylamitriptyline had a detectable effect on Kv when added to the intracellular solution. 6. It is concluded that the uncharged form of amitriptyline is approximately one hundred times more potent in blocking Kv than the charged form. However, this does not seem to be due to uncharged amitriptyline having better access to an intracellular binding site.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armstrong C. M. Interaction of tetraethylammonium ion derivatives with the potassium channels of giant axons. J Gen Physiol. 1971 Oct;58(4):413–437. doi: 10.1085/jgp.58.4.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardoni R., Belluzzi O. Modifications of A-current kinetics in mammalian central neurones induced by extracellular zinc. J Physiol. 1994 Sep 15;479(Pt 3):389–400. doi: 10.1113/jphysiol.1994.sp020304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beech D. J., Bernheim L., Mathie A., Hille B. Intracellular Ca2+ buffers disrupt muscarinic suppression of Ca2+ current and M current in rat sympathetic neurons. Proc Natl Acad Sci U S A. 1991 Jan 15;88(2):652–656. doi: 10.1073/pnas.88.2.652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernheim L., Beech D. J., Hille B. A diffusible second messenger mediates one of the pathways coupling receptors to calcium channels in rat sympathetic neurons. Neuron. 1991 Jun;6(6):859–867. doi: 10.1016/0896-6273(91)90226-p. [DOI] [PubMed] [Google Scholar]
- Chesler M., Kaila K. Modulation of pH by neuronal activity. Trends Neurosci. 1992 Oct;15(10):396–402. doi: 10.1016/0166-2236(92)90191-a. [DOI] [PubMed] [Google Scholar]
- Choquet D., Korn H. Mechanism of 4-aminopyridine action on voltage-gated potassium channels in lymphocytes. J Gen Physiol. 1992 Feb;99(2):217–240. doi: 10.1085/jgp.99.2.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtney K. R. Mechanism of frequency-dependent inhibition of sodium currents in frog myelinated nerve by the lidocaine derivative GEA. J Pharmacol Exp Ther. 1975 Nov;195(2):225–236. [PubMed] [Google Scholar]
- Ehlert F. J., Delen F. M., Yun S. H., Liem H. A. The interaction of amitriptyline, doxepin, imipramine and their N-methyl quaternary ammonium derivatives with subtypes of muscarinic receptors in brain and heart. J Pharmacol Exp Ther. 1990 Apr;253(1):13–19. [PubMed] [Google Scholar]
- French R. J., Shoukimas J. J. Blockage of squid axon potassium conductance by internal tetra-N-alkylammonium ions of various sizes. Biophys J. 1981 May;34(2):271–291. doi: 10.1016/S0006-3495(81)84849-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halliwell R. F., Peters J. A., Lambert J. J. The mechanism of action and pharmacological specificity of the anticonvulsant NMDA antagonist MK-801: a voltage clamp study on neuronal cells in culture. Br J Pharmacol. 1989 Feb;96(2):480–494. doi: 10.1111/j.1476-5381.1989.tb11841.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Hille B. Potassium channels in myelinated nerve. Selective permeability to small cations. J Gen Physiol. 1973 Jun;61(6):669–686. doi: 10.1085/jgp.61.6.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howe J. R., Ritchie J. M. On the active form of 4-aminopyridine: block of K+ currents in rabbit Schwann cells. J Physiol. 1991 Feb;433:183–205. doi: 10.1113/jphysiol.1991.sp018421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kehl S. J. Quinidine-induced inhibition of the fast transient outward K+ current in rat melanotrophs. Br J Pharmacol. 1991 Jul;103(3):1807–1813. doi: 10.1111/j.1476-5381.1991.tb09867.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotake H., Saitoh M., Ogino K., Hirai S., Matsuoka S., Hasegawa J., Mashiba H. On the ionic mechanism of cyproheptadine-induced bradycardia in a rabbit sinoatrial node preparation. Eur J Pharmacol. 1987 Jul 23;139(3):307–313. doi: 10.1016/0014-2999(87)90588-7. [DOI] [PubMed] [Google Scholar]
- Lowe D. A., Matthews E. K., Richardson B. P. The calcium antagonistic effects of cyproheptadine on contraction, membrane electrical events and calcium influx in the guinea-pig taenia coli. Br J Pharmacol. 1981 Nov;74(3):651–663. doi: 10.1111/j.1476-5381.1981.tb10476.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narahashi T., Frazier D. T. Site of action and active form of procaine in squid giant axons. J Pharmacol Exp Ther. 1975 Sep;194(3):506–513. [PubMed] [Google Scholar]
- Pusch M., Neher E. Rates of diffusional exchange between small cells and a measuring patch pipette. Pflugers Arch. 1988 Feb;411(2):204–211. doi: 10.1007/BF00582316. [DOI] [PubMed] [Google Scholar]
- Rettig J., Heinemann S. H., Wunder F., Lorra C., Parcej D. N., Dolly J. O., Pongs O. Inactivation properties of voltage-gated K+ channels altered by presence of beta-subunit. Nature. 1994 May 26;369(6478):289–294. doi: 10.1038/369289a0. [DOI] [PubMed] [Google Scholar]
- Riccioppo Neto F. Effects of cyproheptadine on electrophysiological properties of isolated cardiac muscle of dogs and rabbits. Br J Pharmacol. 1983 Oct;80(2):335–341. doi: 10.1111/j.1476-5381.1983.tb10038.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins J., Sim J. A. A transient outward current in NG108-15 neuroblastoma x glioma hybrid cells. Pflugers Arch. 1990 Apr;416(1-2):130–137. doi: 10.1007/BF00370234. [DOI] [PubMed] [Google Scholar]
- Stephens G. J., Garratt J. C., Robertson B., Owen D. G. On the mechanism of 4-aminopyridine action on the cloned mouse brain potassium channel mKv1.1. J Physiol. 1994 Jun 1;477(Pt 2):187–196. doi: 10.1113/jphysiol.1994.sp020183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strichartz G. R. The inhibition of sodium currents in myelinated nerve by quaternary derivatives of lidocaine. J Gen Physiol. 1973 Jul;62(1):37–57. doi: 10.1085/jgp.62.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talukder G., Harrison N. L. On the mechanism of modulation of transient outward current in cultured rat hippocampal neurons by di- and trivalent cations. J Neurophysiol. 1995 Jan;73(1):73–79. doi: 10.1152/jn.1995.73.1.73. [DOI] [PubMed] [Google Scholar]
- Várnai P., Osipenko O. N., Vizi E. S., Spät A. Activation of calcium current in voltage-clamped rat glomerulosa cells by potassium ions. J Physiol. 1995 Feb 15;483(Pt 1):67–78. doi: 10.1113/jphysiol.1995.sp020568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkins C. S., Mathie A. Modulation of the gating of the transient outward potassium current of rat isolated cerebellar granule neurons by lanthanum. Pflugers Arch. 1994 Oct;428(3-4):209–216. doi: 10.1007/BF00724499. [DOI] [PubMed] [Google Scholar]
- Wong E. H., Kemp J. A., Priestley T., Knight A. R., Woodruff G. N., Iversen L. L. The anticonvulsant MK-801 is a potent N-methyl-D-aspartate antagonist. Proc Natl Acad Sci U S A. 1986 Sep;83(18):7104–7108. doi: 10.1073/pnas.83.18.7104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wooltorton J. R., Mathie A. Block of potassium currents in rat isolated sympathetic neurones by tricyclic antidepressants and structurally related compounds. Br J Pharmacol. 1993 Nov;110(3):1126–1132. doi: 10.1111/j.1476-5381.1993.tb13931.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zagotta W. N., Hoshi T., Aldrich R. W. Restoration of inactivation in mutants of Shaker potassium channels by a peptide derived from ShB. Science. 1990 Oct 26;250(4980):568–571. doi: 10.1126/science.2122520. [DOI] [PubMed] [Google Scholar]
- Zhang Z. H., Follmer C. H., Sarma J. S., Chen F., Singh B. N. Effect of ambasilide, a new class III agent, on plateau currents in isolated guinea pig ventricular myocytes: block of delayed outward potassium current. J Pharmacol Exp Ther. 1992 Oct;263(1):40–48. [PubMed] [Google Scholar]


