Abstract
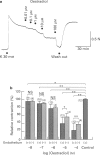
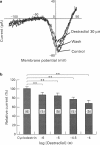
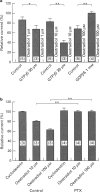
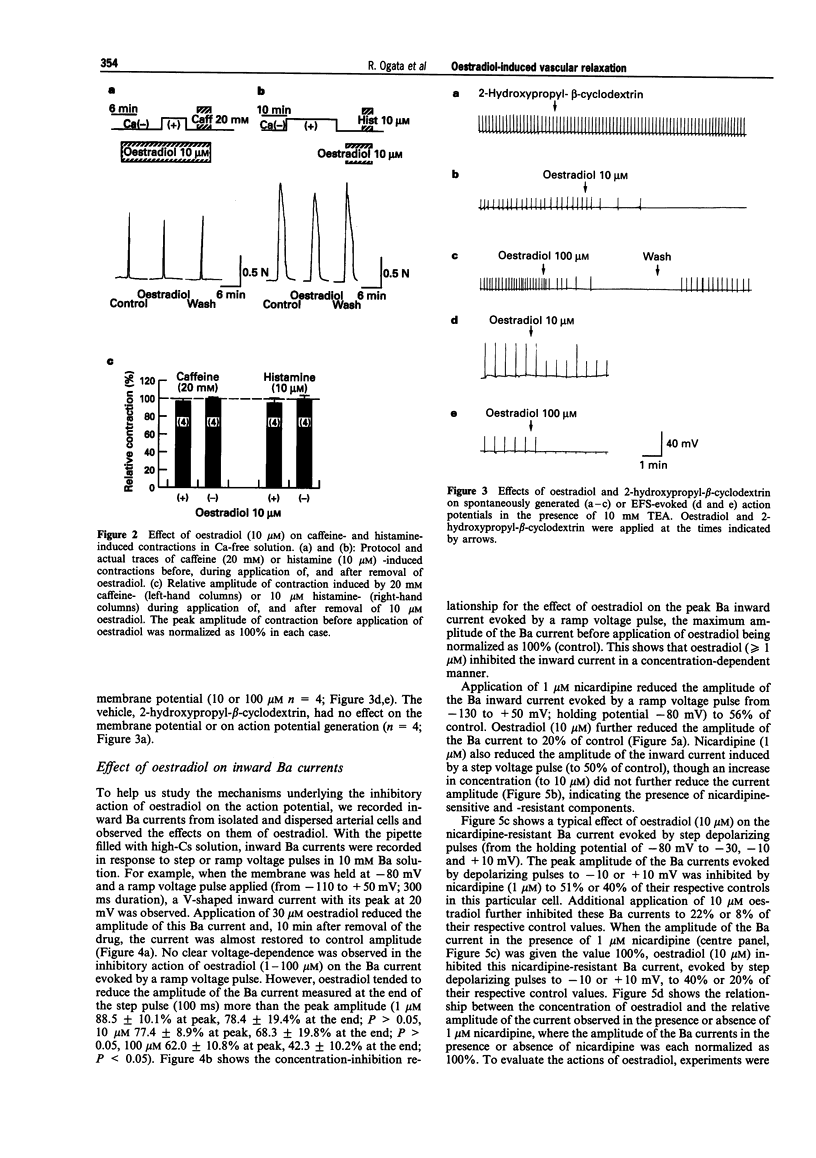
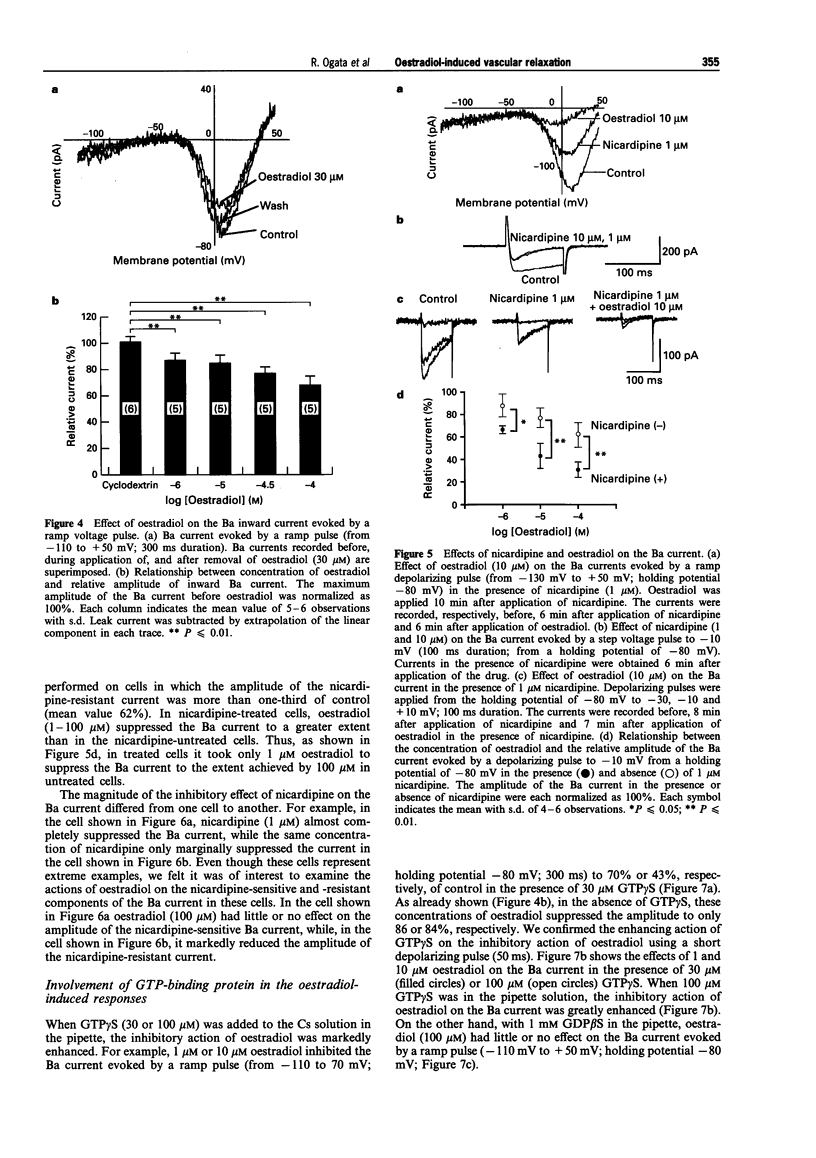
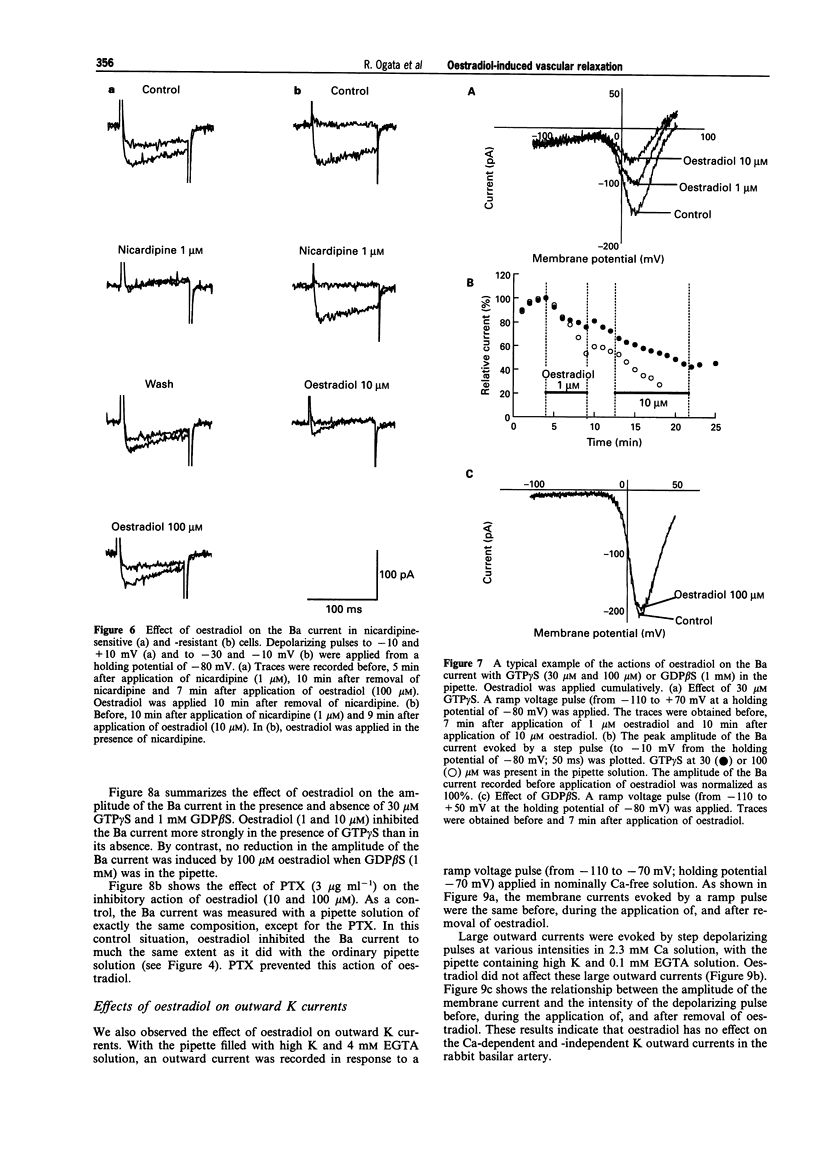
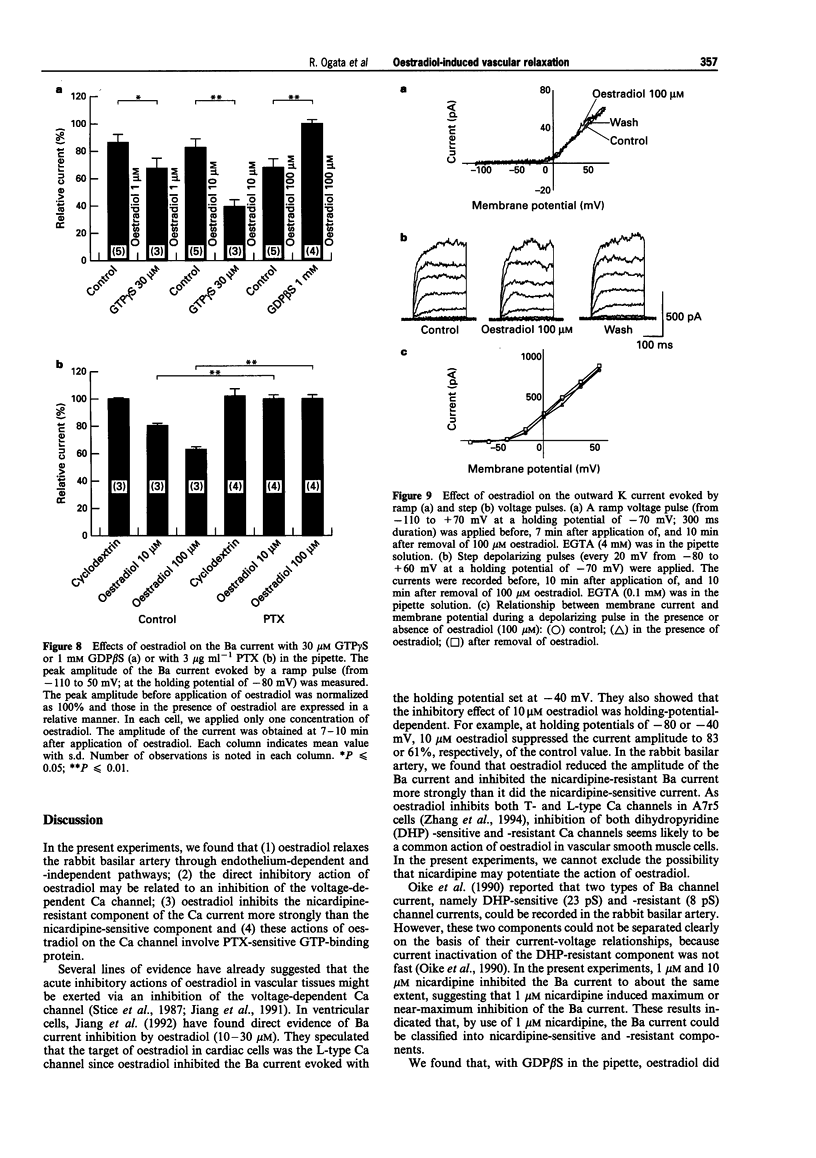
1. Effects of oestradiol on the electrical and mechanical properties of the rabbit basilar artery were investigated by use of microelectrode, patch-clamp and isometric tension recording methods. 2. Oestradiol (10 nM-100 microM) relaxed arterial tissue pre-contracted by excess [K]o solution (30 mM) in a concentration-dependent manner. In Ca-free solution, histamine (10 microM) and caffeine (20 mM) each produced a phasic contraction, but oestradiol (10 microM) did not significantly affect their amplitude. 3. Oestradiol (< or = 100 microM) did not change the resting membrane potential of the artery whether in the presence or absence of TEA (10 mM). Action potentials observed in the presence of 10 mM TEA were abolished by oestradiol (100 microM). 4. Oestradiol (1 microM-100 microM) inhibited the voltage-dependent Ba current in a concentration-dependent manner. Oestradiol (100 microM) inhibited the Ba current observed in the presence of nicardipine (1 microM) more than that in the absence of nicardipine (to 31.0% vs 62.0% of control). 5. GTP gamma S (30 microM) in the pipette enhanced the inhibitory actions of oestradiol on the Ba current. On the other hand, with GDP beta S (1 mM) in the pipette, oestradiol failed to inhibit the Ba current. Pertussis toxin (PTX 3 micrograms ml-1) in the pipette totally prevented the inhibitory action of oestradiol on the Ba current. 6. Oestradiol (< or = 100 microM) had no significant effect on the outward K currents evoked by a membrane depolarization. 7. These results strongly suggest that oestradiol relaxes arterial tissue by inhibition of voltage-dependent Ca channels and that it inhibits both nicardipine-sensitive and -resistant Ca currents via a PTX-sensitive GTP-binding protein. The main target of oestradiol among the arterial Ca channels seems to be the nicardipine-resistant Ca channel, rather than the nicardipine-sensitive one.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benham C. D., Tsien R. W. Noradrenaline modulation of calcium channels in single smooth muscle cells from rabbit ear artery. J Physiol. 1988 Oct;404:767–784. doi: 10.1113/jphysiol.1988.sp017318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bush T. L., Barrett-Connor E., Cowan L. D., Criqui M. H., Wallace R. B., Suchindran C. M., Tyroler H. A., Rifkind B. M. Cardiovascular mortality and noncontraceptive use of estrogen in women: results from the Lipid Research Clinics Program Follow-up Study. Circulation. 1987 Jun;75(6):1102–1109. doi: 10.1161/01.cir.75.6.1102. [DOI] [PubMed] [Google Scholar]
- Collins P., Rosano G. M., Jiang C., Lindsay D., Sarrel P. M., Poole-Wilson P. A. Cardiovascular protection by oestrogen--a calcium antagonist effect? Lancet. 1993 May 15;341(8855):1264–1265. doi: 10.1016/0140-6736(93)91158-i. [DOI] [PubMed] [Google Scholar]
- Droogmans G., Declerck I., Casteels R. Effect of adrenergic agonists on Ca2+-channel currents in single vascular smooth muscle cells. Pflugers Arch. 1987 Jun;409(1-2):7–12. doi: 10.1007/BF00584744. [DOI] [PubMed] [Google Scholar]
- Girasole G., Jilka R. L., Passeri G., Boswell S., Boder G., Williams D. C., Manolagas S. C. 17 beta-estradiol inhibits interleukin-6 production by bone marrow-derived stromal cells and osteoblasts in vitro: a potential mechanism for the antiosteoporotic effect of estrogens. J Clin Invest. 1992 Mar;89(3):883–891. doi: 10.1172/JCI115668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gisclard V., Miller V. M., Vanhoutte P. M. Effect of 17 beta-estradiol on endothelium-dependent responses in the rabbit. J Pharmacol Exp Ther. 1988 Jan;244(1):19–22. [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Han S. Z., Karaki H., Ouchi Y., Akishita M., Orimo H. 17 beta-Estradiol inhibits Ca2+ influx and Ca2+ release induced by thromboxane A2 in porcine coronary artery. Circulation. 1995 May 15;91(10):2619–2626. doi: 10.1161/01.cir.91.10.2619. [DOI] [PubMed] [Google Scholar]
- Henderson B. E., Paganini-Hill A., Ross R. K. Estrogen replacement therapy and protection from acute myocardial infarction. Am J Obstet Gynecol. 1988 Aug;159(2):312–317. doi: 10.1016/s0002-9378(88)80074-7. [DOI] [PubMed] [Google Scholar]
- Jiang C. W., Sarrel P. M., Lindsay D. C., Poole-Wilson P. A., Collins P. Endothelium-independent relaxation of rabbit coronary artery by 17 beta-oestradiol in vitro. Br J Pharmacol. 1991 Dec;104(4):1033–1037. doi: 10.1111/j.1476-5381.1991.tb12545.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang C., Poole-Wilson P. A., Sarrel P. M., Mochizuki S., Collins P., MacLeod K. T. Effect of 17 beta-oestradiol on contraction, Ca2+ current and intracellular free Ca2+ in guinea-pig isolated cardiac myocytes. Br J Pharmacol. 1992 Jul;106(3):739–745. doi: 10.1111/j.1476-5381.1992.tb14403.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knopp R. H. The effects of postmenopausal estrogen therapy on the incidence of arteriosclerotic vascular disease. Obstet Gynecol. 1988 Nov;72(5 Suppl):23S–30S. [PubMed] [Google Scholar]
- Loirand G., Pacaud P., Mironneau C., Mironneau J. GTP-binding proteins mediate noradrenaline effects on calcium and chloride currents in rat portal vein myocytes. J Physiol. 1990 Sep;428:517–529. doi: 10.1113/jphysiol.1990.sp018225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magness R. R., Rosenfeld C. R. Local and systemic estradiol-17 beta: effects on uterine and systemic vasodilation. Am J Physiol. 1989 Apr;256(4 Pt 1):E536–E542. doi: 10.1152/ajpendo.1989.256.4.E536. [DOI] [PubMed] [Google Scholar]
- Matsuda S., Kadowaki Y., Ichino M., Akiyama T., Toyoshima K., Yamamoto T. 17 beta-estradiol mimics ligand activity of the c-erbB2 protooncogene product. Proc Natl Acad Sci U S A. 1993 Nov 15;90(22):10803–10807. doi: 10.1073/pnas.90.22.10803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oike M., Inoue Y., Kitamura K., Kuriyama H. Dual action of FRC8653, a novel dihydropyridine derivative, on the Ba2+ current recorded from the rabbit basilar artery. Circ Res. 1990 Oct;67(4):993–1006. doi: 10.1161/01.res.67.4.993. [DOI] [PubMed] [Google Scholar]
- Oike M., Kitamura K., Kuriyama H. Histamine H3-receptor activation augments voltage-dependent Ca2+ current via GTP hydrolysis in rabbit saphenous artery. J Physiol. 1992 Mar;448:133–152. doi: 10.1113/jphysiol.1992.sp019033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacaud P., Loirand G., Mironneau C., Mironneau J. Opposing effects of noradrenaline on the two classes of voltage-dependent calcium channels of single vascular smooth muscle cells in short-term primary culture. Pflugers Arch. 1987 Nov;410(4-5):557–559. doi: 10.1007/BF00586539. [DOI] [PubMed] [Google Scholar]
- Paganini-Hill A., Ross R. K., Henderson B. E. Postmenopausal oestrogen treatment and stroke: a prospective study. BMJ. 1988 Aug 20;297(6647):519–522. doi: 10.1136/bmj.297.6647.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pines A., Fisman E. Z., Levo Y., Averbuch M., Lidor A., Drory Y., Finkelstein A., Hetman-Peri M., Moshkowitz M., Ben-Ari E. The effects of hormone replacement therapy in normal postmenopausal women: measurements of Doppler-derived parameters of aortic flow. Am J Obstet Gynecol. 1991 Mar;164(3):806–812. doi: 10.1016/0002-9378(91)90520-2. [DOI] [PubMed] [Google Scholar]
- Shamma F. N., Fayad P., Brass L., Sarrel P. Middle cerebral artery blood velocity during controlled ovarian hyperstimulation. Fertil Steril. 1992 May;57(5):1022–1025. [PubMed] [Google Scholar]
- Stampfer M. J., Colditz G. A., Willett W. C., Manson J. E., Rosner B., Speizer F. E., Hennekens C. H. Postmenopausal estrogen therapy and cardiovascular disease. Ten-year follow-up from the nurses' health study. N Engl J Med. 1991 Sep 12;325(11):756–762. doi: 10.1056/NEJM199109123251102. [DOI] [PubMed] [Google Scholar]
- Stampfer M. J., Willett W. C., Colditz G. A., Rosner B., Speizer F. E., Hennekens C. H. A prospective study of postmenopausal estrogen therapy and coronary heart disease. N Engl J Med. 1985 Oct 24;313(17):1044–1049. doi: 10.1056/NEJM198510243131703. [DOI] [PubMed] [Google Scholar]
- Stice S. L., Ford S. P., Rosazza J. P., Van Orden D. E. Role of 4-hydroxylated estradiol in reducing Ca2+ uptake of uterine arterial smooth muscle cells through potential-sensitive channels. Biol Reprod. 1987 Mar;36(2):361–368. doi: 10.1095/biolreprod36.2.361. [DOI] [PubMed] [Google Scholar]
- Williams J. K., Adams M. R., Klopfenstein H. S. Estrogen modulates responses of atherosclerotic coronary arteries. Circulation. 1990 May;81(5):1680–1687. doi: 10.1161/01.cir.81.5.1680. [DOI] [PubMed] [Google Scholar]
- Xiong Z. L., Kitamura K., Kuriyama H. ATP activates cationic currents and modulates the calcium current through GTP-binding protein in rabbit portal vein. J Physiol. 1991;440:143–165. doi: 10.1113/jphysiol.1991.sp018701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang F., Ram J. L., Standley P. R., Sowers J. R. 17 beta-Estradiol attenuates voltage-dependent Ca2+ currents in A7r5 vascular smooth muscle cell line. Am J Physiol. 1994 Apr;266(4 Pt 1):C975–C980. doi: 10.1152/ajpcell.1994.266.4.C975. [DOI] [PubMed] [Google Scholar]