Abstract
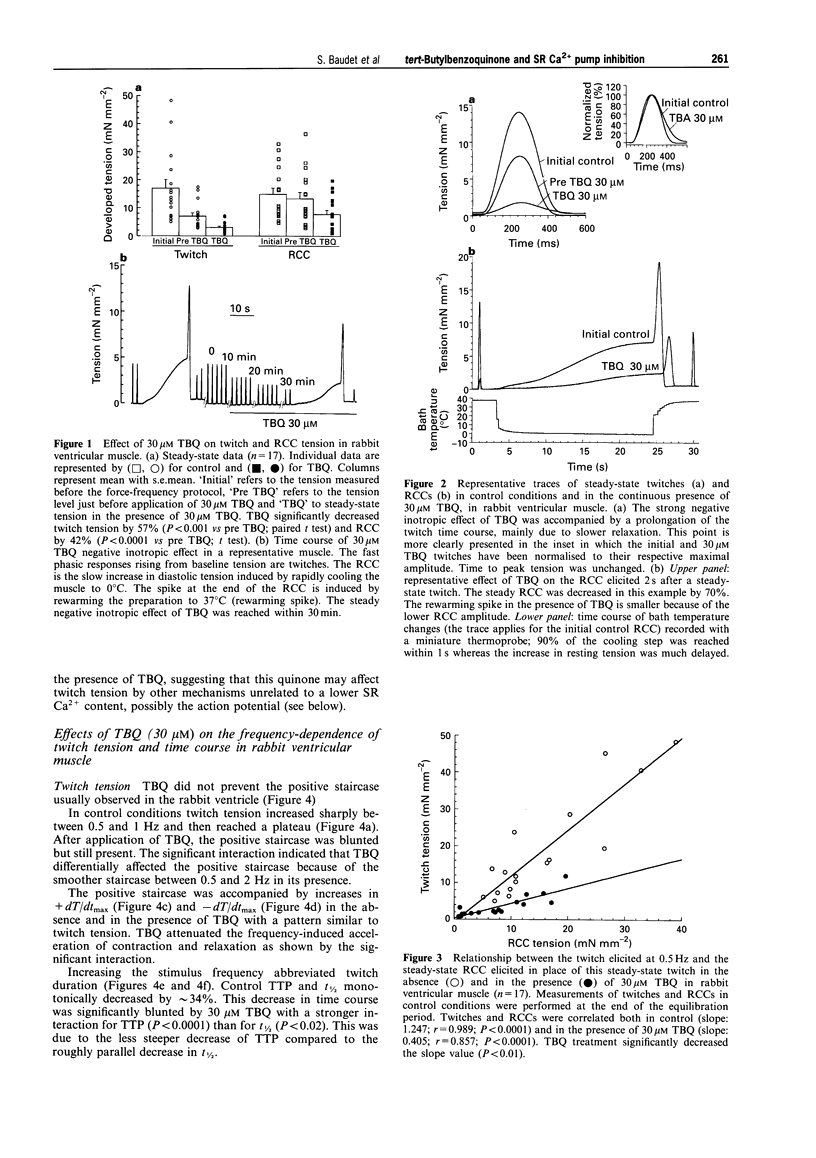
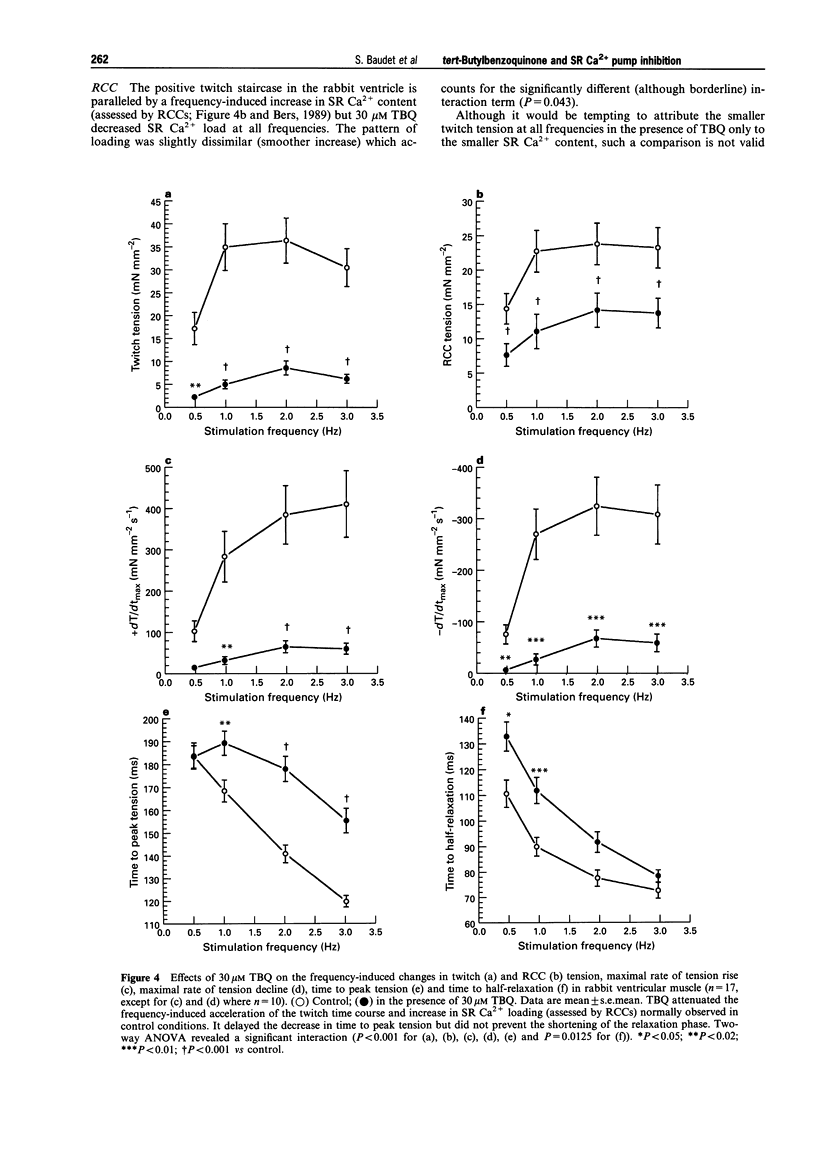
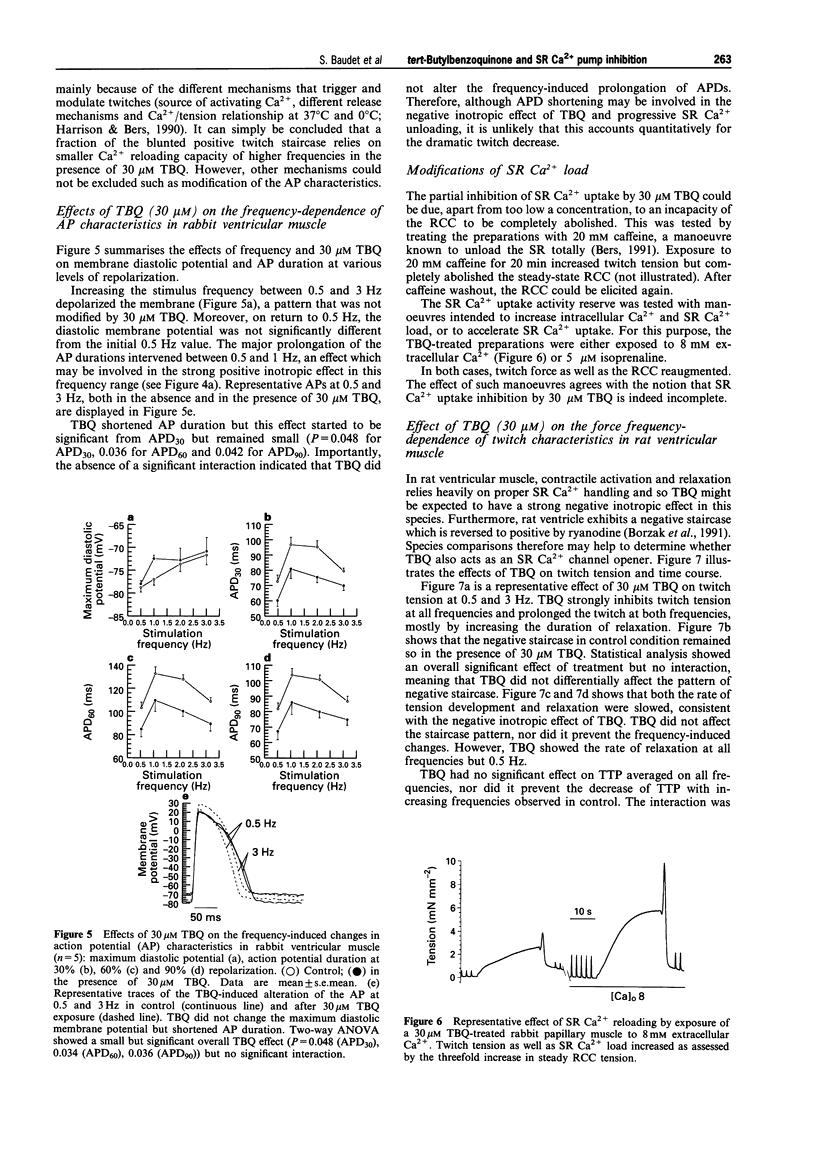
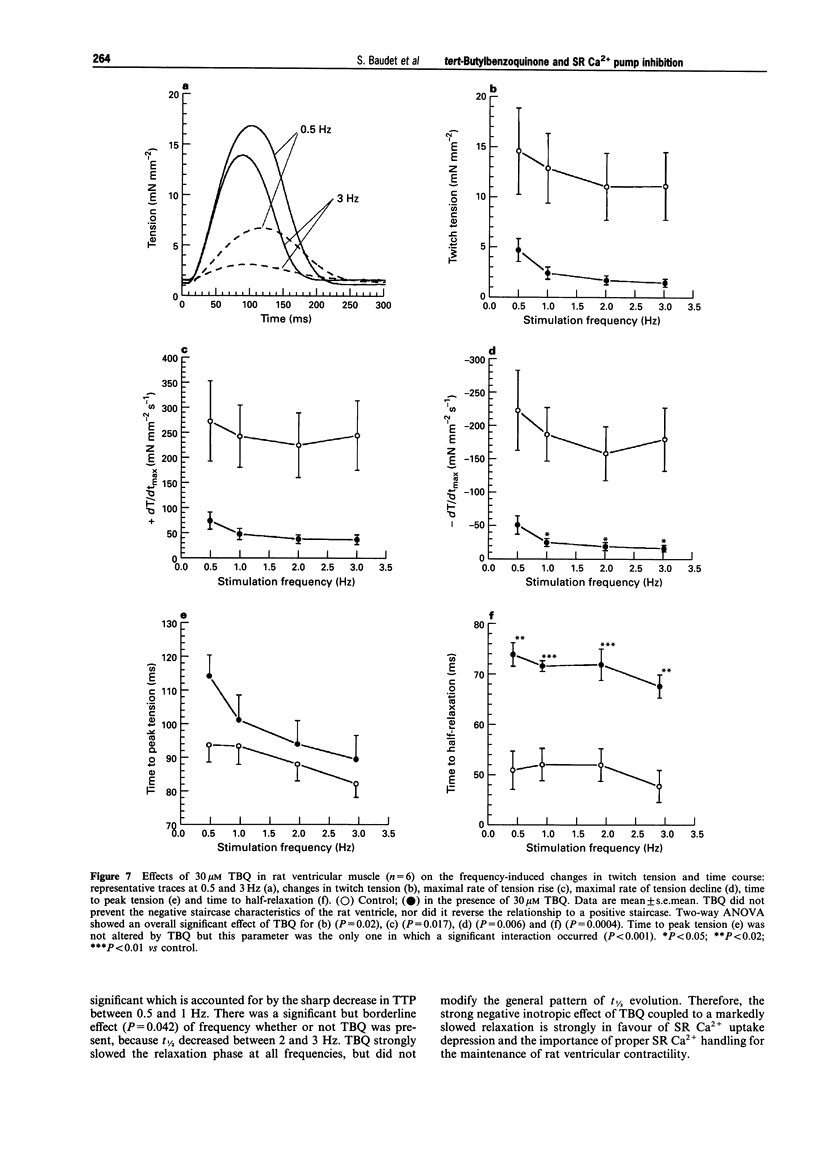
1. The effects of 2,5 di-(tert-butyl)-1,4-benzohydroquinone (TBQ), a putative inhibitor of the sarcoplasmic reticulum (SR) Ca2+ pump, on twitch tension, time course and SR Ca2+ content have been studied at different stimulation frequencies (0.5-3 Hz) in isolated preparations from the rabbit and rat right ventricle, at 37 degrees C. 2. At 0.5Hz, 30 microM TBQ induced a marked negative inotropic effect in both species (-57% in the rabbit and -68% in the rat) and decreased the rate of rise and fall of twitch tension. In parallel, SR Ca2+ content (assessed by rapid cooling contractures) was depressed in the rabbit by 42%. The force-frequency relationship (positive for the rabbit and negative for the rat) was significantly attenuated. In the rabbit, this alteration was shown to rely on insufficient SR Ca2+ reloading with increasing frequencies. 3. Exposure of TBQ-treated preparations to 8 mM extracellular Ca2+ or 5 microM isoprenaline were effective in reloading the SR with Ca2+ whereas 20 mM caffeine emptied this compartment. 4. In the rabbit ventricle, increase in stimulation frequency shortened control twitch time course by decreasing both the time to peak tension (TTP) and the time to half relaxation (t1/2). TBQ did not differentially affect the pattern for t1/2 but significantly attenuated the frequency-induced decrease of TTP. 5. In rabbit ventricular muscle, the action potential duration increased between 0.5 and 3 Hz whether or not TBQ was present. However, TBQ induced a small but significant additional action potential shortening. 6. TBQ decreased twitch tension in the rat ventricle between 0.5 and 3 Hz but the negative staircase was not differentially affected by the SR Ca2+ pump inhibitor. In control conditions and in the presence of 30 microM TBQ, t1/2 was frequency-independent but TBQ consistently increased this parameter (by approximately 29%). 7. These data argue in favour of a specific and partial inhibition of the SR Ca2+ pump by 30 microM TBQ in the rabbit and rat ventricle and emphasise the importance of SR Ca2+ uptake in the force-frequency phenomenon.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arai M., Matsui H., Periasamy M. Sarcoplasmic reticulum gene expression in cardiac hypertrophy and heart failure. Circ Res. 1994 Apr;74(4):555–564. doi: 10.1161/01.res.74.4.555. [DOI] [PubMed] [Google Scholar]
- Backx P. H., Gao W. D., Azan-Backx M. D., Marban E. The relationship between contractile force and intracellular [Ca2+] in intact rat cardiac trabeculae. J Gen Physiol. 1995 Jan;105(1):1–19. doi: 10.1085/jgp.105.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barry W. H., Bridge J. H. Intracellular calcium homeostasis in cardiac myocytes. Circulation. 1993 Jun;87(6):1806–1815. doi: 10.1161/01.cir.87.6.1806. [DOI] [PubMed] [Google Scholar]
- Bassani J. W., Bassani R. A., Bers D. M. Relaxation in rabbit and rat cardiac cells: species-dependent differences in cellular mechanisms. J Physiol. 1994 Apr 15;476(2):279–293. doi: 10.1113/jphysiol.1994.sp020130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassani J. W., Bassani R. A., Bers D. M. Twitch-dependent SR Ca accumulation and release in rabbit ventricular myocytes. Am J Physiol. 1993 Aug;265(2 Pt 1):C533–C540. doi: 10.1152/ajpcell.1993.265.2.C533. [DOI] [PubMed] [Google Scholar]
- Bassani R. A., Mattiazzi A., Bers D. M. CaMKII is responsible for activity-dependent acceleration of relaxation in rat ventricular myocytes. Am J Physiol. 1995 Feb;268(2 Pt 2):H703–H712. doi: 10.1152/ajpheart.1995.268.2.H703. [DOI] [PubMed] [Google Scholar]
- Baudet S., Shaoulian R., Bers D. M. Effects of thapsigargin and cyclopiazonic acid on twitch force and sarcoplasmic reticulum Ca2+ content of rabbit ventricular muscle. Circ Res. 1993 Nov;73(5):813–819. doi: 10.1161/01.res.73.5.813. [DOI] [PubMed] [Google Scholar]
- Borzak S., Murphy S., Marsh J. D. Mechanisms of rate staircase in rat ventricular cells. Am J Physiol. 1991 Mar;260(3 Pt 2):H884–H892. doi: 10.1152/ajpheart.1991.260.3.H884. [DOI] [PubMed] [Google Scholar]
- Brutsaert D. L., Sys S. U. Relaxation and diastole of the heart. Physiol Rev. 1989 Oct;69(4):1228–1315. doi: 10.1152/physrev.1989.69.4.1228. [DOI] [PubMed] [Google Scholar]
- Busselen P., Bosteels S., Tunstall J. Caffeine rapid cooling contractures and negative force staircase in rat papillary muscle. J Mol Cell Cardiol. 1991 Nov;23(11):1313–1322. doi: 10.1016/0022-2828(91)90088-4. [DOI] [PubMed] [Google Scholar]
- Chemla D., Lecarpentier Y., Martin J. L., Clergue M., Antonetti A., Hatt P. Y. Relationship between inotropy and relaxation in rat myocardium. Am J Physiol. 1986 Jun;250(6 Pt 2):H1008–H1016. doi: 10.1152/ajpheart.1986.250.6.H1008. [DOI] [PubMed] [Google Scholar]
- Du G. G., Ashley C. C., Lea T. J. Effects of thapsigargin and cyclopiazonic acid on the sarcoplasmic reticulum Ca2+ pump of skinned fibres from frog skeletal muscle. Pflugers Arch. 1994 Dec;429(2):169–175. doi: 10.1007/BF00374309. [DOI] [PubMed] [Google Scholar]
- Frampton J. E., Harrison S. M., Boyett M. R., Orchard C. H. Ca2+ and Na+ in rat myocytes showing different force-frequency relationships. Am J Physiol. 1991 Nov;261(5 Pt 1):C739–C750. doi: 10.1152/ajpcell.1991.261.5.C739. [DOI] [PubMed] [Google Scholar]
- Gwathmey J. K., Morgan J. P. Sarcoplasmic reticulum calcium mobilization in right ventricular pressure-overload hypertrophy in the ferret: relationships to diastolic dysfunction and a negative treppe. Pflugers Arch. 1993 Mar;422(6):599–608. doi: 10.1007/BF00374008. [DOI] [PubMed] [Google Scholar]
- Gwathmey J. K., Warren S. E., Briggs G. M., Copelas L., Feldman M. D., Phillips P. J., Callahan M., Jr, Schoen F. J., Grossman W., Morgan J. P. Diastolic dysfunction in hypertrophic cardiomyopathy. Effect on active force generation during systole. J Clin Invest. 1991 Mar;87(3):1023–1031. doi: 10.1172/JCI115061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison S. M., Bers D. M. Temperature dependence of myofilament Ca sensitivity of rat, guinea pig, and frog ventricular muscle. Am J Physiol. 1990 Feb;258(2 Pt 1):C274–C281. doi: 10.1152/ajpcell.1990.258.2.C274. [DOI] [PubMed] [Google Scholar]
- Hasenfuss G., Reinecke H., Studer R., Meyer M., Pieske B., Holtz J., Holubarsch C., Posival H., Just H., Drexler H. Relation between myocardial function and expression of sarcoplasmic reticulum Ca(2+)-ATPase in failing and nonfailing human myocardium. Circ Res. 1994 Sep;75(3):434–442. doi: 10.1161/01.res.75.3.434. [DOI] [PubMed] [Google Scholar]
- Inesi G., Sagara Y. Specific inhibitors of intracellular Ca2+ transport ATPases. J Membr Biol. 1994 Jul;141(1):1–6. doi: 10.1007/BF00232868. [DOI] [PubMed] [Google Scholar]
- Janczewski A. M., Lakatta E. G. Buffering of calcium influx by sarcoplasmic reticulum during the action potential in guinea-pig ventricular myocytes. J Physiol. 1993 Nov;471:343–363. doi: 10.1113/jphysiol.1993.sp019904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitakaze M., Weisman H. F., Marban E. Contractile dysfunction and ATP depletion after transient calcium overload in perfused ferret hearts. Circulation. 1988 Mar;77(3):685–695. doi: 10.1161/01.cir.77.3.685. [DOI] [PubMed] [Google Scholar]
- Kiyosue T., Arita M., Muramatsu H., Spindler A. J., Noble D. Ionic mechanisms of action potential prolongation at low temperature in guinea-pig ventricular myocytes. J Physiol. 1993 Aug;468:85–106. doi: 10.1113/jphysiol.1993.sp019761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewartowski B., Pytkowski B. Cellular mechanism of the relationship between myocardial force and frequency of contractions. Prog Biophys Mol Biol. 1987;50(2):97–120. doi: 10.1016/0079-6107(87)90005-8. [DOI] [PubMed] [Google Scholar]
- Lewartowski B., Rózycka M., Janiak R. Effects of thapsigargin in normal and pretreated with ryanodine guinea pig cardiomyocytes. Am J Physiol. 1994 May;266(5 Pt 2):H1829–H1839. doi: 10.1152/ajpheart.1994.266.5.H1829. [DOI] [PubMed] [Google Scholar]
- Liu C. P., Ting C. T., Lawrence W., Maughan W. L., Chang M. S., Kass D. A. Diminished contractile response to increased heart rate in intact human left ventricular hypertrophy. Systolic versus diastolic determinants. Circulation. 1993 Oct;88(4 Pt 1):1893–1906. doi: 10.1161/01.cir.88.4.1893. [DOI] [PubMed] [Google Scholar]
- Llopis J., Chow S. B., Kass G. E., Gahm A., Orrenius S. Comparison between the effects of the microsomal Ca(2+)-translocase inhibitors thapsigargin and 2,5-di-(t-butyl)-1,4-benzohydroquinone on cellular calcium fluxes. Biochem J. 1991 Jul 15;277(Pt 2):553–556. doi: 10.1042/bj2770553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludbrook J. Repeated measurements and multiple comparisons in cardiovascular research. Cardiovasc Res. 1994 Mar;28(3):303–311. doi: 10.1093/cvr/28.3.303. [DOI] [PubMed] [Google Scholar]
- Mitchell M. R., Powell T., Terrar D. A., Twist V. W. Influence of a change in stimulation rate on action potentials, currents and contractions in rat ventricular cells. J Physiol. 1985 Jul;364:113–130. doi: 10.1113/jphysiol.1985.sp015734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morcos N. C., Gardin J. M., Tomita N., Henry W. L. Improvement of relaxation velocity parameters by calcium channel blockers in the aging rabbit myocardium. Basic Res Cardiol. 1992 Sep-Oct;87(5):437–451. doi: 10.1007/BF00795056. [DOI] [PubMed] [Google Scholar]
- Nakagawa Y., Moldéus P. Cytotoxic effects of phenyl-hydroquinone and some hydroquinones on isolated rat hepatocytes. Biochem Pharmacol. 1992 Sep 25;44(6):1059–1065. doi: 10.1016/0006-2952(92)90368-s. [DOI] [PubMed] [Google Scholar]
- Negretti N., O'Neill S. C., Eisner D. A. The effects of inhibitors of sarcoplasmic reticulum function on the systolic Ca2+ transient in rat ventricular myocytes. J Physiol. 1993 Aug;468:35–52. doi: 10.1113/jphysiol.1993.sp019758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson E. J., Li C. C., Bangalore R., Benson T., Kass R. S., Hinkle P. M. Inhibition of L-type calcium-channel activity by thapsigargin and 2,5-t-butylhydroquinone, but not by cyclopiazonic acid. Biochem J. 1994 Aug 15;302(Pt 1):147–154. doi: 10.1042/bj3020147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pery-Man N., Chemla D., Coirault C., Suard I., Riou B., Lecarpentier Y. A comparison of cyclopiazonic acid and ryanodine effects on cardiac muscle relaxation. Am J Physiol. 1993 Oct;265(4 Pt 2):H1364–H1372. doi: 10.1152/ajpheart.1993.265.4.H1364. [DOI] [PubMed] [Google Scholar]
- Schmidt U., Schwinger R. H., Böhm M., Erdmann E. Alterations of the force-frequency relation depending on stages of heart failure in humans. Am J Cardiol. 1994 Nov 15;74(10):1066–1068. doi: 10.1016/0002-9149(94)90862-1. [DOI] [PubMed] [Google Scholar]
- Schouten V. J., ter Keurs H. E. Role of Ica and Na+/Ca2+ exchange in the force-frequency relationship of rat heart muscle. J Mol Cell Cardiol. 1991 Sep;23(9):1039–1050. doi: 10.1016/0022-2828(91)91639-9. [DOI] [PubMed] [Google Scholar]
- Sellin L. C., McArdle J. J. Multiple effects of 2,3-butanedione monoxime. Pharmacol Toxicol. 1994 Jun;74(6):305–313. doi: 10.1111/j.1600-0773.1994.tb01365.x. [DOI] [PubMed] [Google Scholar]
- Shattock M. J., Bers D. M. Rat vs. rabbit ventricle: Ca flux and intracellular Na assessed by ion-selective microelectrodes. Am J Physiol. 1989 Apr;256(4 Pt 1):C813–C822. doi: 10.1152/ajpcell.1989.256.4.C813. [DOI] [PubMed] [Google Scholar]
- Studer R., Reinecke H., Bilger J., Eschenhagen T., Böhm M., Hasenfuss G., Just H., Holtz J., Drexler H. Gene expression of the cardiac Na(+)-Ca2+ exchanger in end-stage human heart failure. Circ Res. 1994 Sep;75(3):443–453. doi: 10.1161/01.res.75.3.443. [DOI] [PubMed] [Google Scholar]
- Westerblad H., Allen D. G. The role of sarcoplasmic reticulum in relaxation of mouse muscle; effects of 2,5-di(tert-butyl)-1,4-benzohydroquinone. J Physiol. 1994 Jan 15;474(2):291–301. doi: 10.1113/jphysiol.1994.sp020022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wrzosek A., Schneider H., Grueninger S., Chiesi M. Effect of thapsigargin on cardiac muscle cells. Cell Calcium. 1992 May;13(5):281–292. doi: 10.1016/0143-4160(92)90063-x. [DOI] [PubMed] [Google Scholar]
- Yard N. J., Chiesi M., Ball H. A. Effect of cyclopiazonic acid, an inhibitor of sarcoplasmic reticulum Ca(2+)-ATPase, on the frequency-dependence of the contraction-relaxation cycle of the guinea-pig isolated atrium. Br J Pharmacol. 1994 Nov;113(3):1001–1007. doi: 10.1111/j.1476-5381.1994.tb17092.x. [DOI] [PMC free article] [PubMed] [Google Scholar]



