Abstract
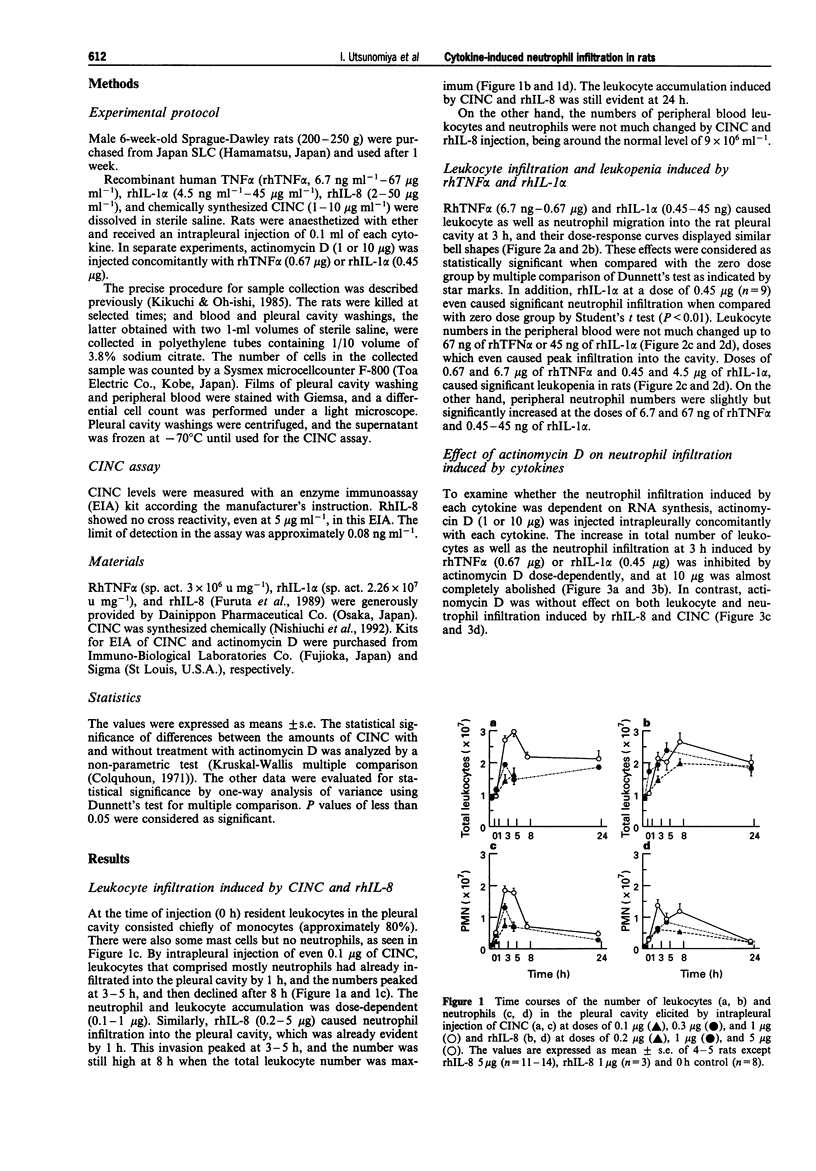
1. To assess in vivo chemotactic activity of tumour necrosis factor (TNF), interleukin-1 (IL-1), IL-8, and cytokine-induced neutrophil chemoattractant (CINC), we injected these cytokines into the pleural cavity of rats. 2. CINC (0.1-1 microgram) and recombinant human IL-8 (rhIL-8, 0.2-5 micrograms) caused neutrophil infiltration into the rat pleural cavity in a dose-dependent fashion, peaking at 3 h. The number of leukocytes in the peripheral blood did not change significantly. 3. RhTNF alpha and rhIL-1 alpha also induced neutrophil accumulation. The dose response curves of rhTNF alpha (0.67 ng-6.7 micrograms) and rhIL-1 alpha (0.45 ng-4.5 micrograms) at 3 h were bell shaped. On the other hand, unlike CINC and rhIL-8, rhTNF alpha and rhIL-1 alpha caused transient marked leukopenia at 3 h in a simple dose-dependent fashion. 4. Concomitant injection of actinomycin D dose-dependently and completely at 10 micrograms inhibited neutrophil infiltration induced by rhTNF alpha (0.67 microgram) and rhIL-1 alpha (0.45 microgram) at 3 h. However, that induced by CINC or rhIL-8 was not affected by actinomycin D. 5. Peaking at 1 h, CINC production in the pleural cavity was found after intrapleural injection of rhTNF alpha (0.67 microgram) or rhIL-1 alpha (0.45 microgram), but not after that of rhIL-8 (5 micrograms). The CINC production induced by rhTNF alpha or rhIL-1 alpha and the neutrophil infiltration was suppressed by concomitant injection of actinomycin D (1 and 10 micrograms). 6. These results indicate that CINC and IL-8 themselves are direct chemoattractants for neutrophils, whereas TNF and IL-1 induce neutrophil infiltration indirectly via newly synthesized mRNA for chemotactic protein including CINC, which may be involved in neutrophil emigration at local inflammatory sites in rats.
Full text
PDF
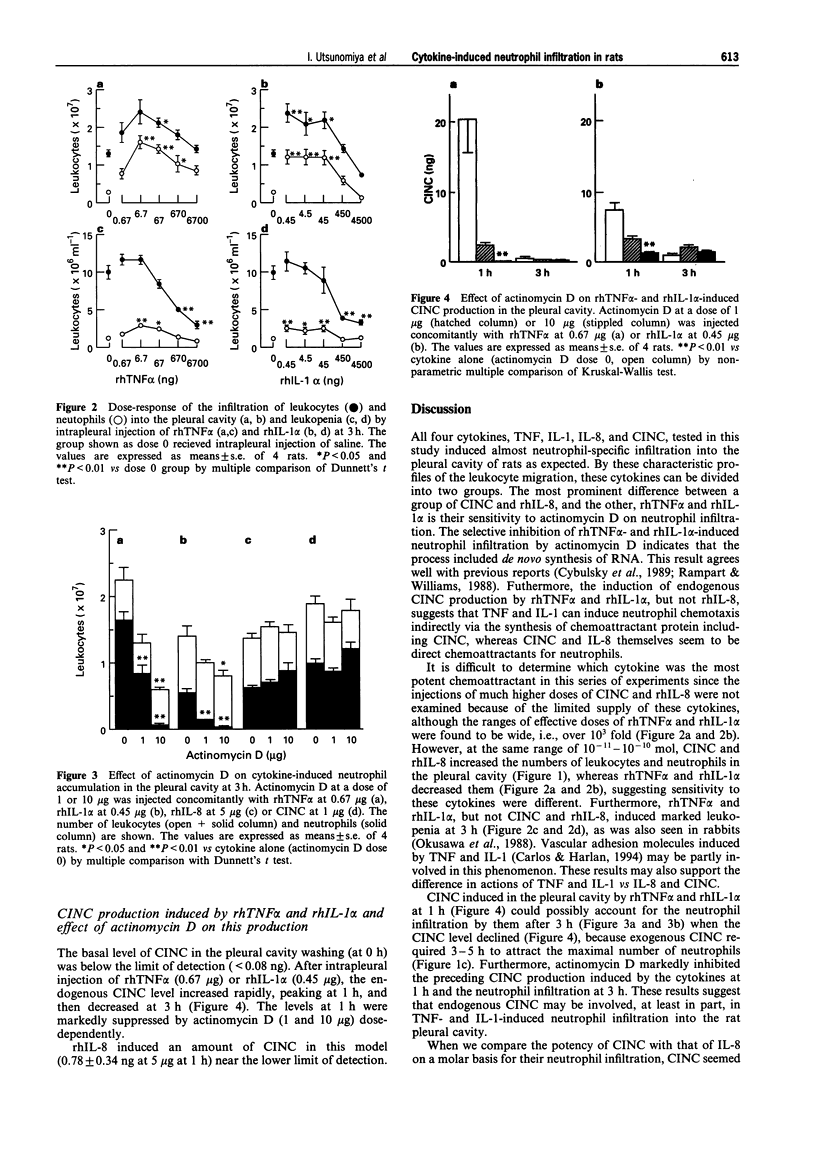

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Balentien E., Han J. H., Thomas H. G., Wen D. Z., Samantha A. K., Zachariae C. O., Griffin P. R., Brachmann R., Wong W. L., Matsushima K. Recombinant expression, biochemical characterization, and biological activities of the human MGSA/gro protein. Biochemistry. 1990 Nov 6;29(44):10225–10233. doi: 10.1021/bi00496a011. [DOI] [PubMed] [Google Scholar]
- Carlos T. M., Harlan J. M. Leukocyte-endothelial adhesion molecules. Blood. 1994 Oct 1;84(7):2068–2101. [PubMed] [Google Scholar]
- Cybulsky M. I., McComb D. J., Movat H. Z. Protein synthesis dependent and independent mechanisms of neutrophil emigration. Different mechanisms of inflammation in rabbits induced by interleukin-1, tumor necrosis factor alpha or endotoxin versus leukocyte chemoattractants. Am J Pathol. 1989 Jul;135(1):227–237. [PMC free article] [PubMed] [Google Scholar]
- Furuta R., Yamagishi J., Kotani H., Sakamoto F., Fukui T., Matsui Y., Sohmura Y., Yamada M., Yoshimura T., Larsen C. G. Production and characterization of recombinant human neutrophil chemotactic factor. J Biochem. 1989 Sep;106(3):436–441. doi: 10.1093/oxfordjournals.jbchem.a122870. [DOI] [PubMed] [Google Scholar]
- Harada A., Sekido N., Kuno K., Akiyama M., Kasahara T., Nakanishi I., Mukaida N., Matsushima K. Expression of recombinant rabbit IL-8 in Escherichia coli and establishment of the essential involvement of IL-8 in recruiting neutrophils into lipopolysaccharide-induced inflammatory site of rabbit skin. Int Immunol. 1993 Jun;5(6):681–690. doi: 10.1093/intimm/5.6.681. [DOI] [PubMed] [Google Scholar]
- Hirasawa N., Watanabe M., Mue S., Watanabe K., Tsurufuji S., Ohuchi K. Induction of neutrophil infiltration by rat chemotactic cytokine (CINC) and its inhibition by dexamethasone in rats. Inflammation. 1992 Apr;16(2):187–196. doi: 10.1007/BF00918958. [DOI] [PubMed] [Google Scholar]
- Kikuchi M., Oh-ishi S. Involvement of histamine in vascular permeability increase of the rat pleurisy induced by phorbol myristate acetate. Jpn J Pharmacol. 1985 Dec;39(4):467–473. doi: 10.1254/jjp.39.467. [DOI] [PubMed] [Google Scholar]
- Larsen C. G., Anderson A. O., Appella E., Oppenheim J. J., Matsushima K. The neutrophil-activating protein (NAP-1) is also chemotactic for T lymphocytes. Science. 1989 Mar 17;243(4897):1464–1466. doi: 10.1126/science.2648569. [DOI] [PubMed] [Google Scholar]
- Le J., Vilcek J. Tumor necrosis factor and interleukin 1: cytokines with multiple overlapping biological activities. Lab Invest. 1987 Mar;56(3):234–248. [PubMed] [Google Scholar]
- Matsushima K., Morishita K., Yoshimura T., Lavu S., Kobayashi Y., Lew W., Appella E., Kung H. F., Leonard E. J., Oppenheim J. J. Molecular cloning of a human monocyte-derived neutrophil chemotactic factor (MDNCF) and the induction of MDNCF mRNA by interleukin 1 and tumor necrosis factor. J Exp Med. 1988 Jun 1;167(6):1883–1893. doi: 10.1084/jem.167.6.1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mrowietz U., Schröder J. M., Christophers E. Recombinant human tumor necrosis factor alpha lacks chemotactic activity for human peripheral blood neutrophils and monocytes. Biochem Biophys Res Commun. 1988 Jun 30;153(3):1223–1228. doi: 10.1016/s0006-291x(88)81358-5. [DOI] [PubMed] [Google Scholar]
- Okusawa S., Gelfand J. A., Ikejima T., Connolly R. J., Dinarello C. A. Interleukin 1 induces a shock-like state in rabbits. Synergism with tumor necrosis factor and the effect of cyclooxygenase inhibition. J Clin Invest. 1988 Apr;81(4):1162–1172. doi: 10.1172/JCI113431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oppenheim J. J., Zachariae C. O., Mukaida N., Matsushima K. Properties of the novel proinflammatory supergene "intercrine" cytokine family. Annu Rev Immunol. 1991;9:617–648. doi: 10.1146/annurev.iy.09.040191.003153. [DOI] [PubMed] [Google Scholar]
- Rampart M., Williams T. J. Evidence that neutrophil accumulation induced by interleukin-1 requires both local protein biosynthesis and neutrophil CD18 antigen expression in vivo. Br J Pharmacol. 1988 Aug;94(4):1143–1148. doi: 10.1111/j.1476-5381.1988.tb11632.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribeiro R. A., Flores C. A., Cunha F. Q., Ferreira S. H. IL-8 causes in vivo neutrophil migration by a cell-dependent mechanism. Immunology. 1991 Aug;73(4):472–477. [PMC free article] [PubMed] [Google Scholar]
- Rot A. Chemotactic potency of recombinant human neutrophil attractant/activation protein-1 (interleukin-8) for polymorphonuclear leukocytes of different species. Cytokine. 1991 Jan;3(1):21–27. doi: 10.1016/1043-4666(91)90006-y. [DOI] [PubMed] [Google Scholar]
- Sekido N., Mukaida N., Harada A., Nakanishi I., Watanabe Y., Matsushima K. Prevention of lung reperfusion injury in rabbits by a monoclonal antibody against interleukin-8. Nature. 1993 Oct 14;365(6447):654–657. doi: 10.1038/365654a0. [DOI] [PubMed] [Google Scholar]
- Utsunomiya I., Nagai S., Oh-ishi S. Differential effects of indomethacin and dexamethasone on cytokine production in carrageenin-induced rat pleurisy. Eur J Pharmacol. 1994 Feb 3;252(2):213–218. doi: 10.1016/0014-2999(94)90599-1. [DOI] [PubMed] [Google Scholar]
- Utsunomiya I., Nagai S., Oh-ishi S. Sequential appearance of IL-1 and IL-6 activities in rat carrageenin-induced pleurisy. J Immunol. 1991 Sep 15;147(6):1803–1809. [PubMed] [Google Scholar]
- Wada T., Tomosugi N., Naito T., Yokoyama H., Kobayashi K., Harada A., Mukaida N., Matsushima K. Prevention of proteinuria by the administration of anti-interleukin 8 antibody in experimental acute immune complex-induced glomerulonephritis. J Exp Med. 1994 Sep 1;180(3):1135–1140. doi: 10.1084/jem.180.3.1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe K., Kinoshita S., Nakagawa H. Purification and characterization of cytokine-induced neutrophil chemoattractant produced by epithelioid cell line of normal rat kidney (NRK-52E cell). Biochem Biophys Res Commun. 1989 Jun 30;161(3):1093–1099. doi: 10.1016/0006-291x(89)91355-7. [DOI] [PubMed] [Google Scholar]
- Watanabe K., Koizumi F., Kurashige Y., Tsurufuji S., Nakagawa H. Rat CINC, a member of the interleukin-8 family, is a neutrophil-specific chemoattractant in vivo. Exp Mol Pathol. 1991 Aug;55(1):30–37. doi: 10.1016/0014-4800(91)90016-q. [DOI] [PubMed] [Google Scholar]
- Watanabe K., Konishi K., Fujioka M., Kinoshita S., Nakagawa H. The neutrophil chemoattractant produced by the rat kidney epithelioid cell line NRK-52E is a protein related to the KC/gro protein. J Biol Chem. 1989 Nov 25;264(33):19559–19563. [PubMed] [Google Scholar]
- Yoshimura T., Matsushima K., Oppenheim J. J., Leonard E. J. Neutrophil chemotactic factor produced by lipopolysaccharide (LPS)-stimulated human blood mononuclear leukocytes: partial characterization and separation from interleukin 1 (IL 1). J Immunol. 1987 Aug 1;139(3):788–793. [PubMed] [Google Scholar]
- Yoshimura T., Matsushima K., Tanaka S., Robinson E. A., Appella E., Oppenheim J. J., Leonard E. J. Purification of a human monocyte-derived neutrophil chemotactic factor that has peptide sequence similarity to other host defense cytokines. Proc Natl Acad Sci U S A. 1987 Dec;84(24):9233–9237. doi: 10.1073/pnas.84.24.9233. [DOI] [PMC free article] [PubMed] [Google Scholar]


