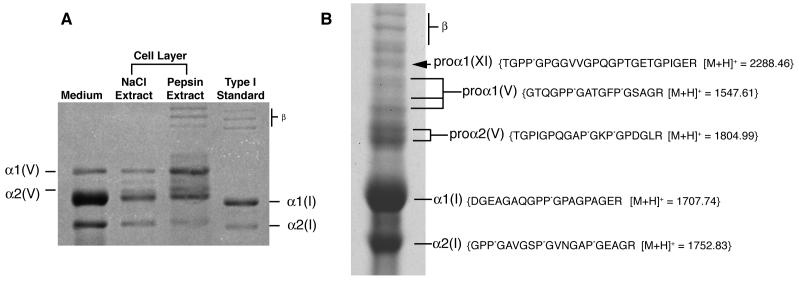
Figure 2.
Biochemical analysis of collagen
A) Collagen types synthesized by the SAOS-2 cell line in culture
The medium and cell layer on pepsin digestion showed types I and V collagen α-chains on SDS-PAGE. The α1(I) and α2(I) chains ran slightly slower than pepsin extracted control preparations from human bone, suggesting post-translational over-modification. Mass spectrometry identified the band between the α1(V) and α2(V) to be a degradation product of the α1(V) chain. Bands marked “β” are dimers of collagen chains.
B) Identification of α1(XI) collagen chains by mass spectrometry
SDS-PAGE (6%) of collagen extracted by 1M NaCl from the extracellular matrix of SAOS-2 cells (+DTT). Arrow indicates a band identified as a pro-form of the α1(XI) collagen chain. The sequence of a tryptic peptide from this band unequivocally identified by mass spectrometry is shown. Mass spectrometry also identified pro-forms of α1(V) and α2(V), as well as processed α1(I) and α2(I) from this gel. Bands marked “β” are dimers of collagen chains.