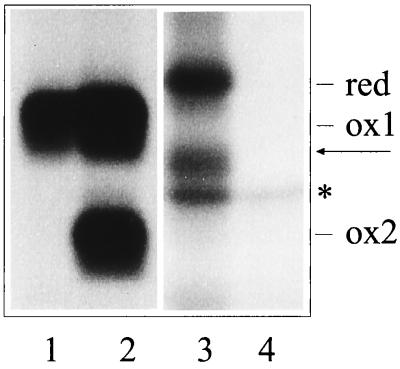
Figure 1.
Identification of the disulfide bond-containing domain in unassembled κNS1 Ig L chains. COS-1 cells were transfected with the various κNS1 cDNAs in a transient expression system. Biosynthetically labeled material was immunoprecipitated by anti-κ-antibodies from the lysate prepared in the presence of NEM from cells that expressed the κNS1V domain mutant (lane 1), the κNS1 wt-Ig L chain (lane 2), the κNS1 C domain mutant (lane 3), or no Ig L chains (lane 4). The migration of the completely reduced (no S—S bond, red), partially oxidized (S—S bond only in the C domain, ox 1), and completely oxidized (S—S bond in both domains, ox2) forms of the Ig L chains is indicated. An additional migration form (arrow) was observed with the C domain mutant and most likely represents a molecule containing a disulfide bond in the V domain. Isolation of the polypeptide marked by an asterisk (∗) is most likely due to nonspecific interactions, as it is also seen in the material precipitated from nontransfected cells (lane 4). Various amounts of this protein coisolated with the different Ig L molecules (i.e., compare lanes 2 and 3). Note that lanes 3 and 4 are from a longer exposure of the autoradiograph, because smaller amounts of labeled material was reproducibly recovered with the C domain mutant.