Abstract
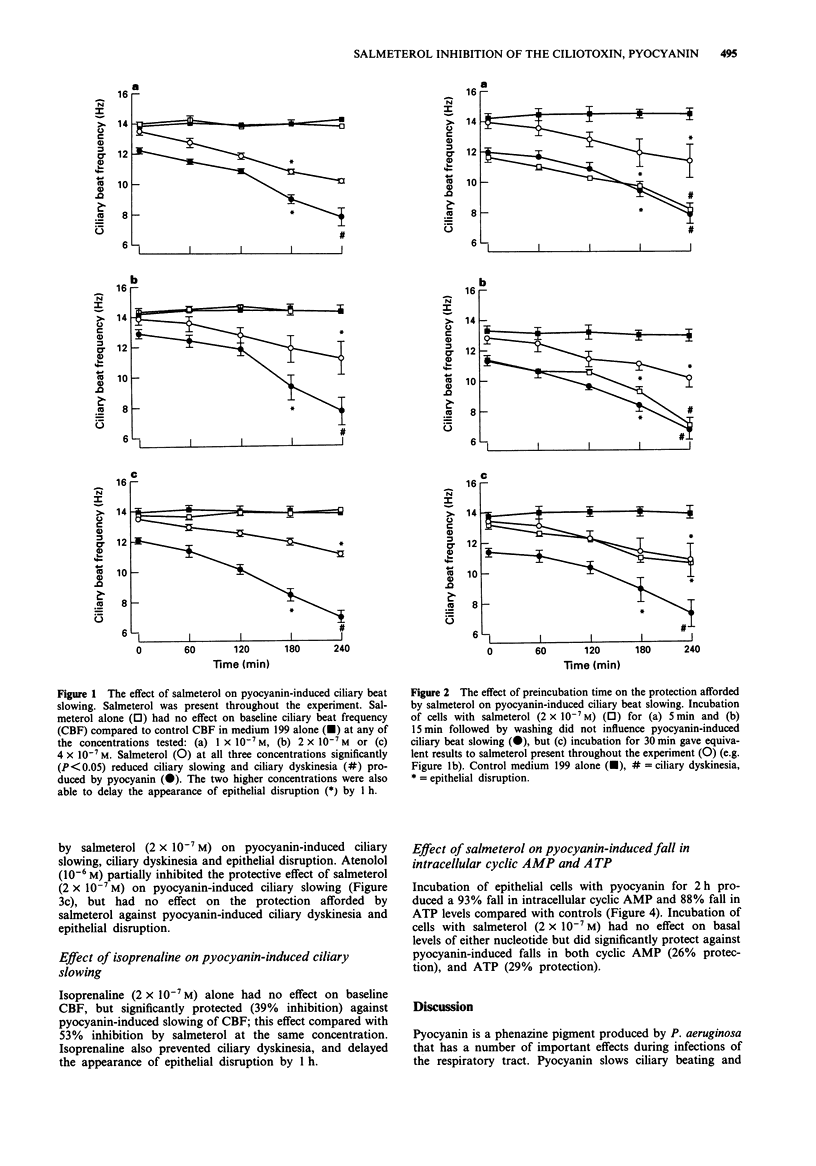
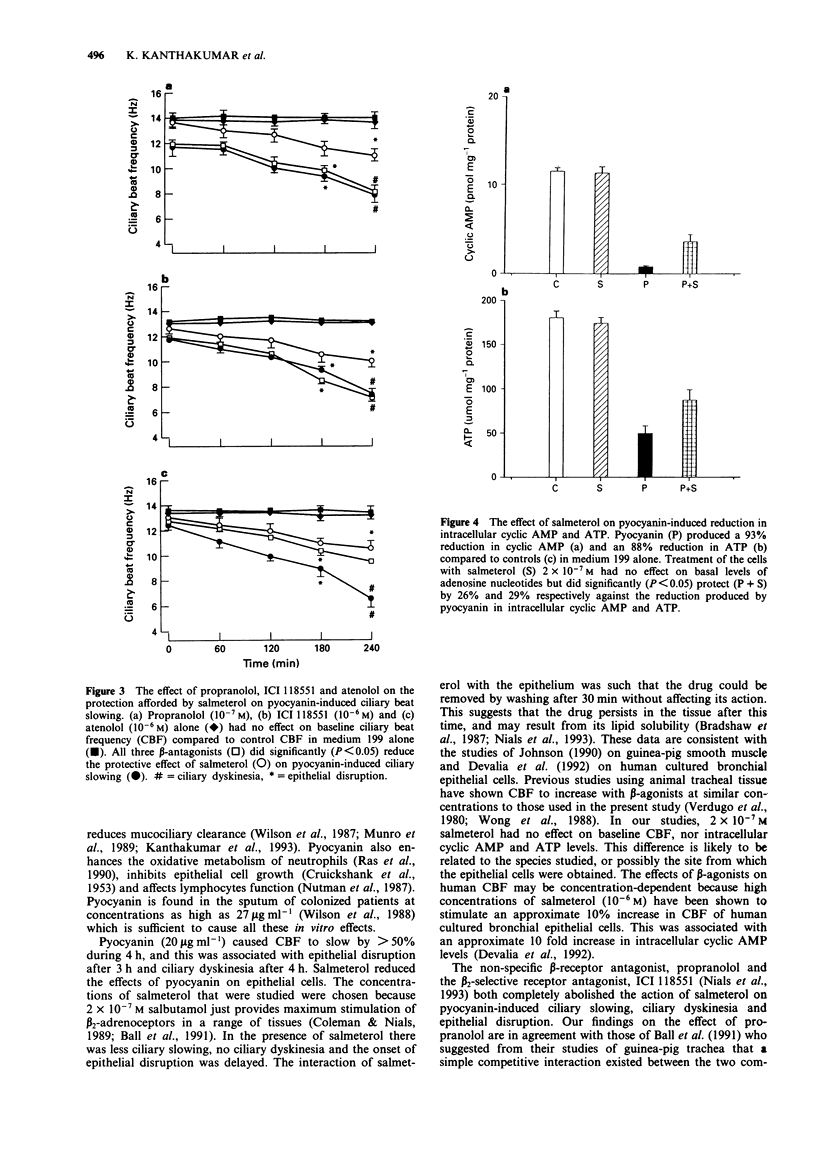
1. Patients with airway infection by Pseudomonas aeruginosa have impaired mucociliary clearance. Pyocyanin is a phenazine pigment produced by P. aeruginosa which is present in the sputum of colonized patients, slows human ciliary beat frequency (CBF) in vitro and slows mucociliary transport in vivo in the guinea-pig. 2. We have investigated the effect of salmeterol, a long-acting beta 2-adrenoceptor agonist, on pyocyanin-induced slowing of human CBF in vitro. Salmeterol (2 x 10(-7) M) was found to reduce pyocycanin (20 micrograms ml-1)-induced slowing of CBF by 53% and the fall in intracellular adenosine 3':5'-cyclic monophosphate (cyclic AMP) by 26% and ATP by 29%. 3. Another beta 2-adrenoceptor agonist, isoprenaline (2 x 10(-7) M), also inhibited pyocyanin-induced slowing of CBF by 39%. 4. The effects of salmeterol (30 min preincubation) persisted after washing the cells. 5. Propranolol (10(-7) M) and the beta 2-specific antagonist, ICI 118551 (10(-6) M) blocked the protective effects of salmeterol completely, but atenolol (10(-6) M) was less effective. These results suggested that the effects of salmeterol on pyocyanin-induced effects were mediated primarily via the stimulation of beta 2-adrenoceptors. 6. Pyocyanin-induced ciliary slowing is associated with a substantial fall in intracellular cyclic AMP and ATP. Salmeterol reversed the effects of pyocyanin on cyclic AMP and ATP. 7. Mucociliary clearance is an important defence mechanism of the airways against bacterial infection. Salmeterol may benefit patients colonized by P. aeruginosa, not only by its bronchodilator action, but also by protecting epithelial cells from pyocyanin-induced slowing of CBF.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ball D. I., Brittain R. T., Coleman R. A., Denyer L. H., Jack D., Johnson M., Lunts L. H., Nials A. T., Sheldrick K. E., Skidmore I. F. Salmeterol, a novel, long-acting beta 2-adrenoceptor agonist: characterization of pharmacological activity in vitro and in vivo. Br J Pharmacol. 1991 Nov;104(3):665–671. doi: 10.1111/j.1476-5381.1991.tb12486.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CRUICKSHANK C. N., LOWBURY E. J. The effect of pyocyanin on human skin cells and leucocytes. Br J Exp Pathol. 1953 Dec;34(6):583–587. [PMC free article] [PubMed] [Google Scholar]
- Coleman R. A., Nials A. T. Novel and versatile superfusion system. Its use in the evaluation of some spasmogenic and spasmolytic agents using guinea-pig isolated tracheal smooth muscle. J Pharmacol Methods. 1989 Mar;21(1):71–86. doi: 10.1016/0160-5402(89)90023-5. [DOI] [PubMed] [Google Scholar]
- Devalia J. L., Sapsford R. J., Rusznak C., Toumbis M. J., Davies R. J. The effects of salmeterol and salbutamol on ciliary beat frequency of cultured human bronchial epithelial cells, in vitro. Pulm Pharmacol. 1992 Dec;5(4):257–263. doi: 10.1016/0952-0600(92)90068-r. [DOI] [PubMed] [Google Scholar]
- Di Benedetto G., Manara-Shediac F. S., Mehta A. Effect of cyclic AMP on ciliary activity of human respiratory epithelium. Eur Respir J. 1991 Jul;4(7):789–795. [PubMed] [Google Scholar]
- Fick R. B., Jr Pathogenesis of the pseudomonas lung lesion in cystic fibrosis. Chest. 1989 Jul;96(1):158–164. doi: 10.1378/chest.96.1.158. [DOI] [PubMed] [Google Scholar]
- Johnson M. The pharmacology of salmeterol. Lung. 1990;168 (Suppl):115–119. doi: 10.1007/BF02718123. [DOI] [PubMed] [Google Scholar]
- Kanthakumar K., Taylor G., Tsang K. W., Cundell D. R., Rutman A., Smith S., Jeffery P. K., Cole P. J., Wilson R. Mechanisms of action of Pseudomonas aeruginosa pyocyanin on human ciliary beat in vitro. Infect Immun. 1993 Jul;61(7):2848–2853. doi: 10.1128/iai.61.7.2848-2853.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight M., Hartman P. E., Hartman Z., Young V. M. A new method of preparation of pyocyanin and demonstration of an unusual bacterial sensitivity. Anal Biochem. 1979 May;95(1):19–23. doi: 10.1016/0003-2697(79)90179-9. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lansley A. B., Sanderson M. J., Dirksen E. R. Control of the beat cycle of respiratory tract cilia by Ca2+ and cAMP. Am J Physiol. 1992 Aug;263(2 Pt 1):L232–L242. doi: 10.1152/ajplung.1992.263.2.L232. [DOI] [PubMed] [Google Scholar]
- Lemoine H., Schönell H., Kaumann A. J. Contribution of beta 1- and beta 2-adrenoceptors of human atrium and ventricle to the effects of noradrenaline and adrenaline as assessed with (-)-atenolol. Br J Pharmacol. 1988 Sep;95(1):55–66. doi: 10.1111/j.1476-5381.1988.tb16548.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munro N. C., Barker A., Rutman A., Taylor G., Watson D., McDonald-Gibson W. J., Towart R., Taylor W. A., Wilson R., Cole P. J. Effect of pyocyanin and 1-hydroxyphenazine on in vivo tracheal mucus velocity. J Appl Physiol (1985) 1989 Jul;67(1):316–323. doi: 10.1152/jappl.1989.67.1.316. [DOI] [PubMed] [Google Scholar]
- Nials A. T., Sumner M. J., Johnson M., Coleman R. A. Investigations into factors determining the duration of action of the beta 2-adrenoceptor agonist, salmeterol. Br J Pharmacol. 1993 Feb;108(2):507–515. doi: 10.1111/j.1476-5381.1993.tb12833.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nutman J., Berger M., Chase P. A., Dearborn D. G., Miller K. M., Waller R. L., Sorensen R. U. Studies on the mechanism of T cell inhibition by the Pseudomonas aeruginosa phenazine pigment pyocyanine. J Immunol. 1987 May 15;138(10):3481–3487. [PubMed] [Google Scholar]
- O'Donnell S. R., Wanstall J. C. Pharmacological approaches to the characterization of beta-adrenoreceptor populations in tissues. J Auton Pharmacol. 1981 Sep;1(4):305–312. doi: 10.1111/j.1474-8673.1981.tb00460.x. [DOI] [PubMed] [Google Scholar]
- O'Donnell S. R., Wanstall J. C. The importance of choice of agonist in studies designed to predict beta 2 : beta 1 adrenoceptor selectivity of antagonists from pA2 values on guinea-pig trachea and atria. Naunyn Schmiedebergs Arch Pharmacol. 1979 Sep;308(3):183–190. doi: 10.1007/BF00501381. [DOI] [PubMed] [Google Scholar]
- Ras G. J., Anderson R., Taylor G. W., Savage J. E., Van Niekerk E., Wilson R., Cole P. J. Proinflammatory interactions of pyocyanin and 1-hydroxyphenazine with human neutrophils in vitro. J Infect Dis. 1990 Jul;162(1):178–185. doi: 10.1093/infdis/162.1.178. [DOI] [PubMed] [Google Scholar]
- Read R. C., Roberts P., Munro N., Rutman A., Hastie A., Shryock T., Hall R., McDonald-Gibson W., Lund V., Taylor G. Effect of Pseudomonas aeruginosa rhamnolipids on mucociliary transport and ciliary beating. J Appl Physiol (1985) 1992 Jun;72(6):2271–2277. doi: 10.1152/jappl.1992.72.6.2271. [DOI] [PubMed] [Google Scholar]
- Rutland J., Cole P. J. Non-invasive sampling of nasal cilia for measurement of beat frequency and study of ultrastructure. Lancet. 1980 Sep 13;2(8194):564–565. doi: 10.1016/s0140-6736(80)91995-9. [DOI] [PubMed] [Google Scholar]
- Satir P., Sleigh M. A. The physiology of cilia and mucociliary interactions. Annu Rev Physiol. 1990;52:137–155. doi: 10.1146/annurev.ph.52.030190.001033. [DOI] [PubMed] [Google Scholar]
- Satir P. The role of axonemal components in ciliary motility. Comp Biochem Physiol A Comp Physiol. 1989;94(2):351–357. doi: 10.1016/0300-9629(89)90558-6. [DOI] [PubMed] [Google Scholar]
- Verdugo P., Johnson N. T., Tam P. Y. beta-Adrenergic stimulation of respiratory ciliary activity. J Appl Physiol Respir Environ Exerc Physiol. 1980 May;48(5):868–871. doi: 10.1152/jappl.1980.48.5.868. [DOI] [PubMed] [Google Scholar]
- Wilson R., Pitt T., Taylor G., Watson D., MacDermot J., Sykes D., Roberts D., Cole P. Pyocyanin and 1-hydroxyphenazine produced by Pseudomonas aeruginosa inhibit the beating of human respiratory cilia in vitro. J Clin Invest. 1987 Jan;79(1):221–229. doi: 10.1172/JCI112787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson R. Secondary ciliary dysfunction. Clin Sci (Lond) 1988 Aug;75(2):113–120. doi: 10.1042/cs0750113. [DOI] [PubMed] [Google Scholar]
- Wilson R., Sykes D. A., Watson D., Rutman A., Taylor G. W., Cole P. J. Measurement of Pseudomonas aeruginosa phenazine pigments in sputum and assessment of their contribution to sputum sol toxicity for respiratory epithelium. Infect Immun. 1988 Sep;56(9):2515–2517. doi: 10.1128/iai.56.9.2515-2517.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong L. B., Miller I. F., Yeates D. B. Regulation of ciliary beat frequency by autonomic mechanisms: in vitro. J Appl Physiol (1985) 1988 Oct;65(4):1895–1901. doi: 10.1152/jappl.1988.65.4.1895. [DOI] [PubMed] [Google Scholar]
- Yeates D. B., Sturgess J. M., Kahn S. R., Levison H., Aspin N. Mucociliary transport in trachea of patients with cystic fibrosis. Arch Dis Child. 1976 Jan;51(1):28–33. doi: 10.1136/adc.51.1.28. [DOI] [PMC free article] [PubMed] [Google Scholar]


