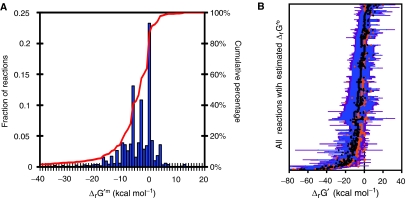
Figure 2.
Thermodynamic properties of the reactions in iAF1260. (A) The distribution of estimated ΔrG′m values for the reactions in iAF1260. ΔrG′m could be estimated for 1996 reactions (96%) in the reconstruction. 64% of the represented reactions have a negative ΔrG′m, and 20% of the reactions have a ΔrG′m of approximately zero. This distribution of ΔrG′m values indicates that most reactions in the model are thermodynamically favorable at millimolar concentration conditions. (B) The range of possible ΔrG′ values for the reactions in iAF1260. ΔrG′ differs from ΔrG′o (orange diamonds) and ΔrG′m (black diamonds) due to variations in metabolite concentrations from the 1 M and 1 mM reference states, respectively. Metabolite concentrations typically range from 0.02 to 0.00001 M, resulting in a wide range of values for ΔrG′ (blue error bars). Taking uncertainty into account, the range of possible values for ΔrG′ can be extended (purple error bars). The ΔrG′ ranges were used to assess the feasibility and reversibility of the reactions in iAF1260; reactions for which a positive ΔrG′ is not possible are thermodynamically irreversible.