Abstract
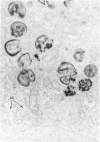
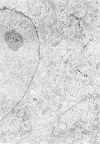
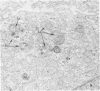
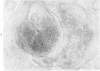
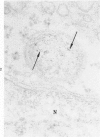
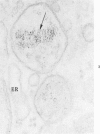
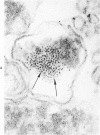
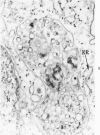
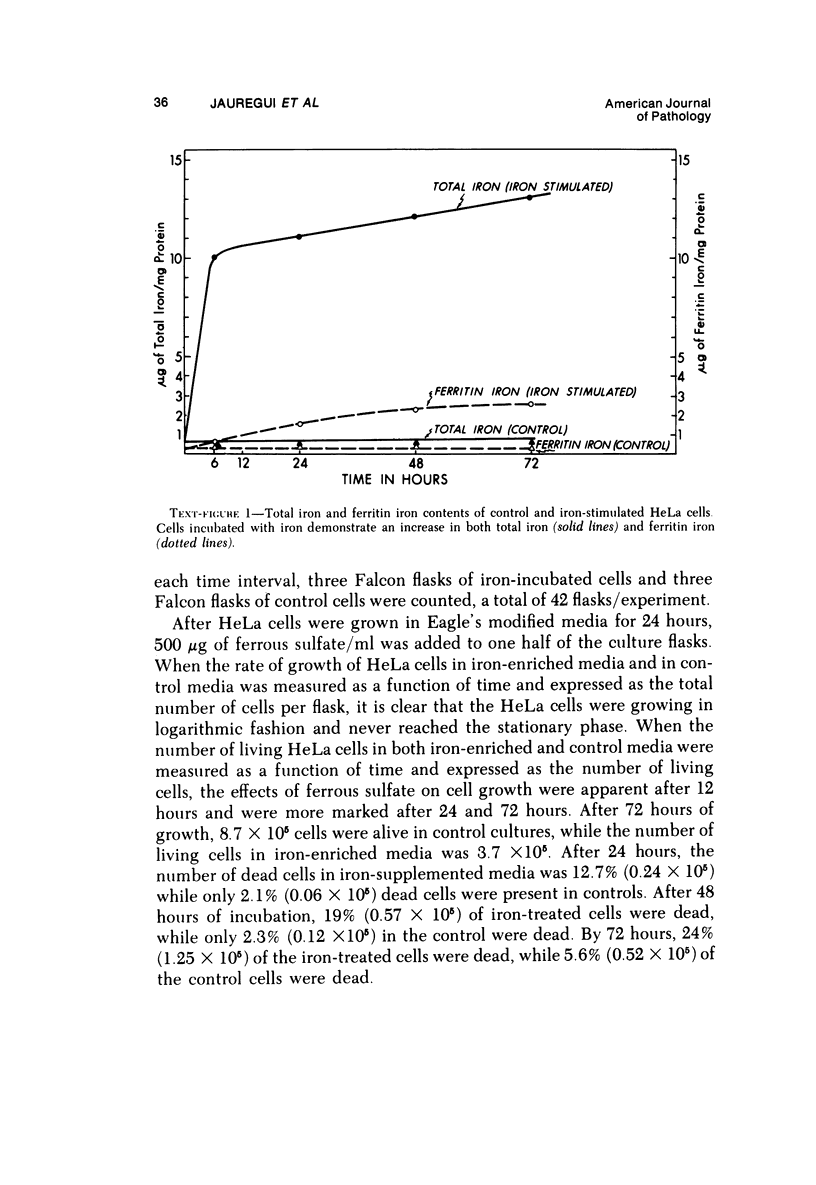
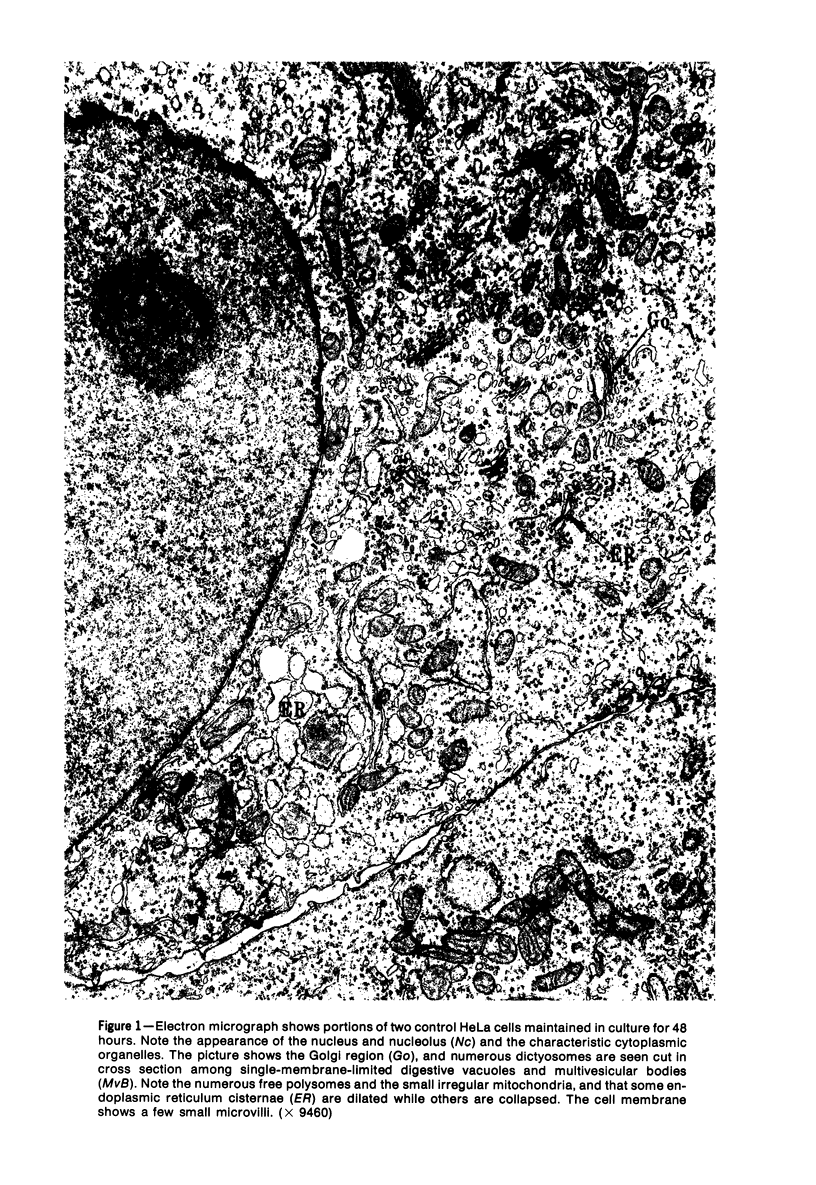
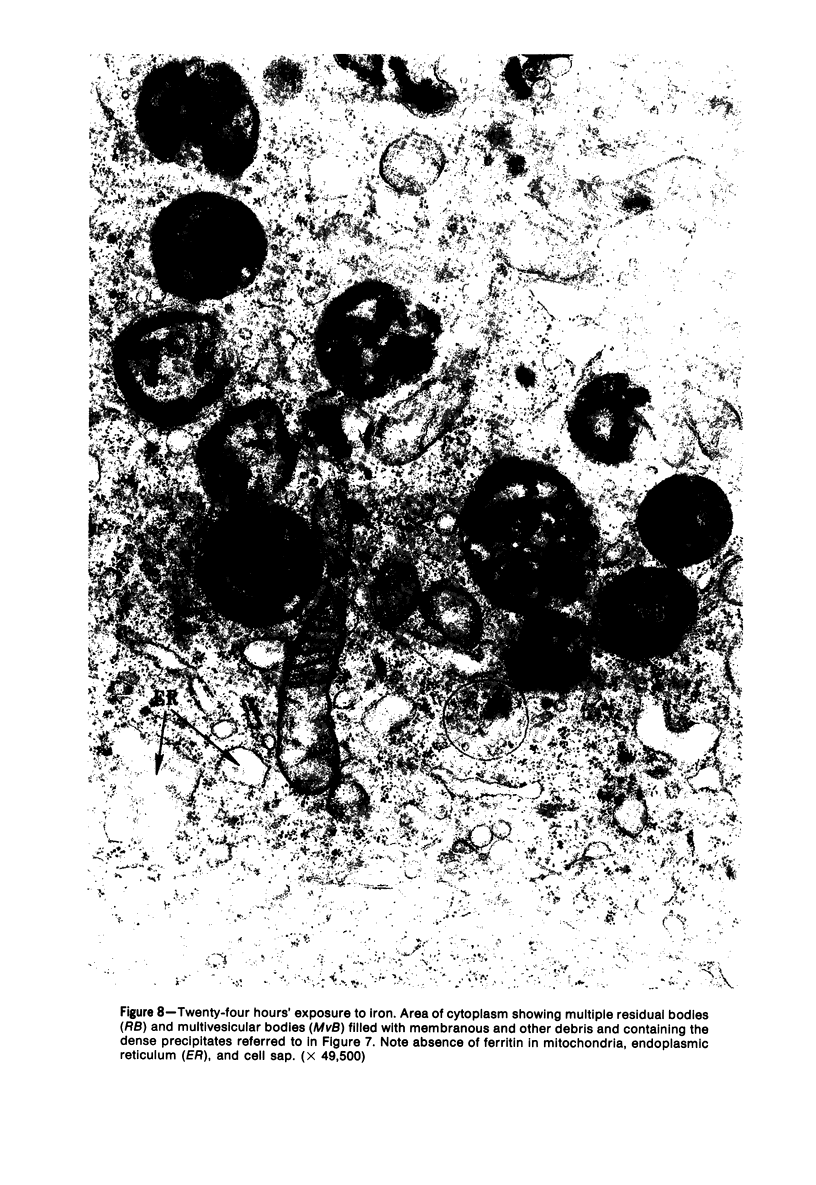
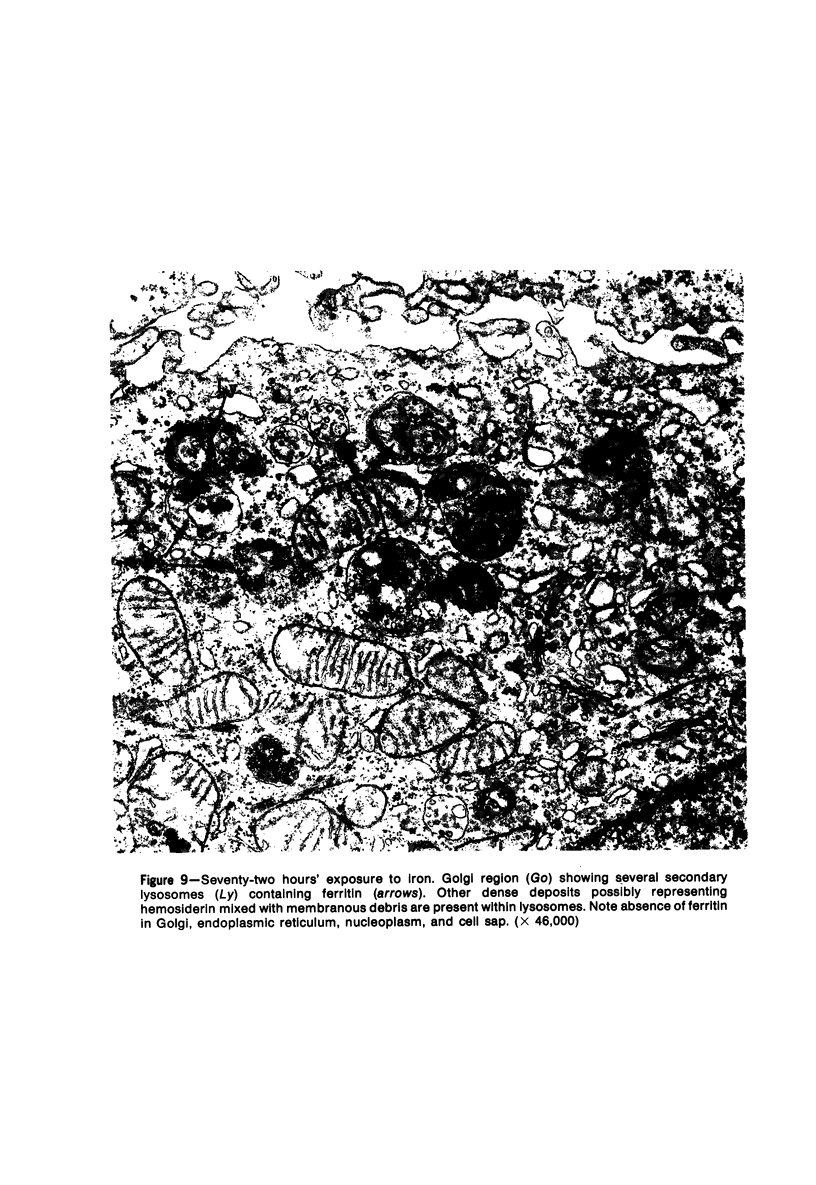
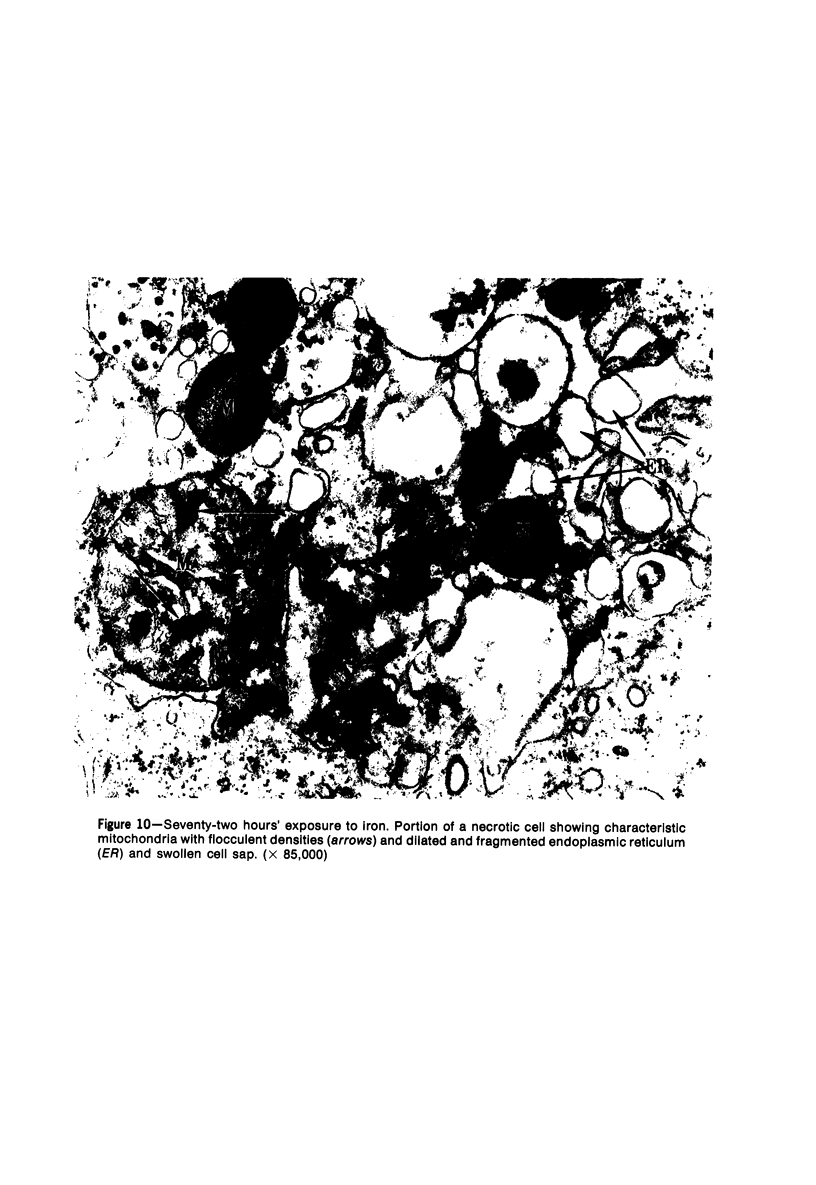
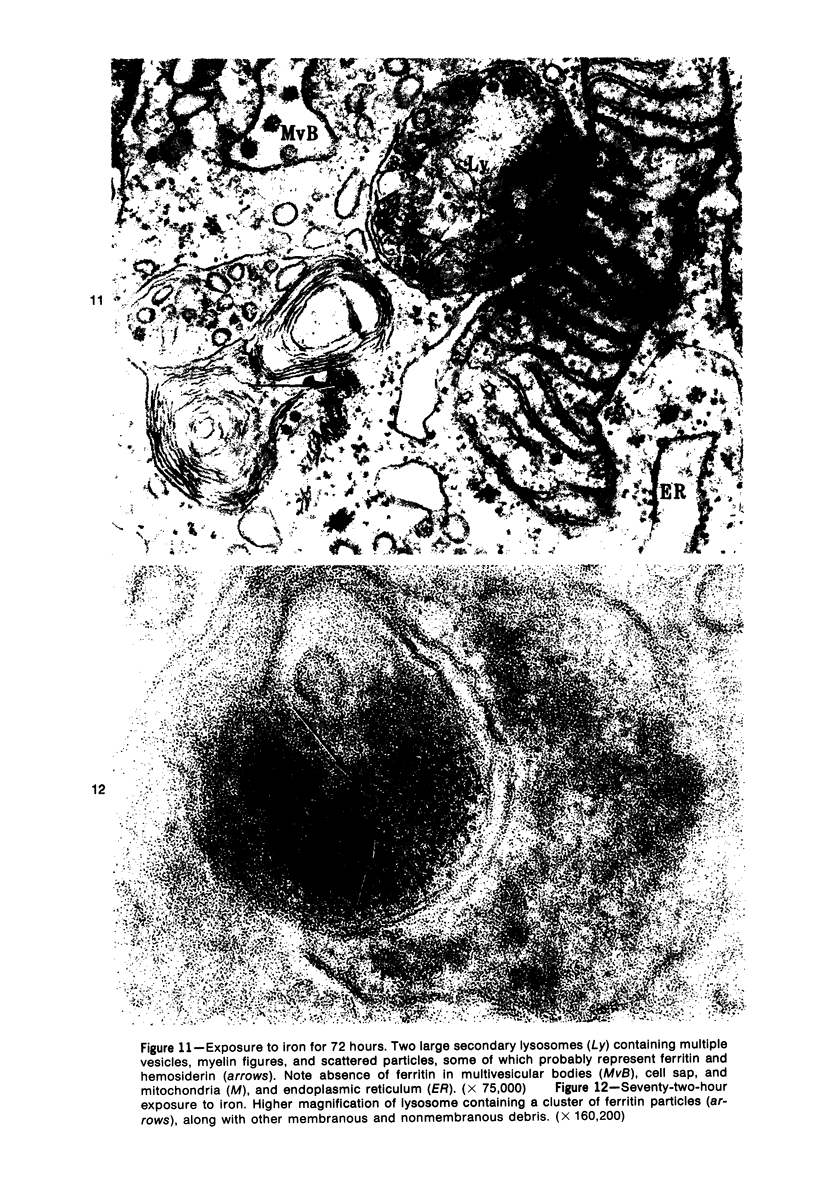
The morphologic characteristics of acute iron loading were studied in HeLa cells incubated in an iron-enriched Eagle's medium containing 500 mug/ml of iron. Chemical studies showed that ferritin synthesis was rapidly induced and the concentration of intracellular ferritin increased up to 72 hours. Closely coupled with an increase in HeLa cell ferritin was a marked decrease in the rate of cell multiplication. The significant ultrastructural findings of iron-induced HeLa cell injury are characterized by the appearance of both autophagic multivesicular and residual bodies over the first 72 hours of iron incubation. The prominence of multivesicular bodies was noted after only 4 hours' incubation, with iron and myelin figures first appearing after 6 hours. Thus, the partial arrest of cell multiplication was associated with an increase in cytoplasmic residual bodies containing iron and other debris. The distribution of intracellular ferritin within HeLa cells differs significantly from the distribution described previously in hepatic parenchymal cells. In HeLa cells, ferritin particles were confined to lysosomal vesicles and were not identified in cell sap, endoplasmic reticulum, or Golgi apparatus.
Full text
PDF











Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arstila A. U., Bradford W. D., Kinney T. D., Trump B. F. Iron metabolism and cell membranes. II. The relationship of ferritin to the cytocavitary network in rat hepatic parenchymal cells. Am J Pathol. 1970 Mar;58(3):419–450. [PMC free article] [PubMed] [Google Scholar]
- Arstila A. U., Jauregui H. O., Chang J., Trump B. F. Studies on cellular autophagocytosis. Relationship between heterophagy and autophagy in HeLa cells. Lab Invest. 1971 Feb;24(2):162–174. [PubMed] [Google Scholar]
- Arstila A. U., Smith M. A., Trump B. F. Microsomal lipid peroxidation: morphological characterization. Science. 1972 Feb 4;175(4021):530–533. doi: 10.1126/science.175.4021.530. [DOI] [PubMed] [Google Scholar]
- Arstila A. U., Trump B. F. Studies on cellular autophagocytosis. The formation of autophagic vacuoles in the liver after glucagon administration. Am J Pathol. 1968 Nov;53(5):687–733. [PMC free article] [PubMed] [Google Scholar]
- Bradford W. D., Elchlepp J. G., Arstila A. U., Trump B. F., Kinney T. D. Iron metabolism and cell membranes. I. Relation between ferritin and hemosiderin in bile and biliary excretion of lysosome contents. Am J Pathol. 1969 Aug;56(2):201–228. [PMC free article] [PubMed] [Google Scholar]
- CONRAD M. E., WEINTRAUB L. R., CROSBY W. H. THE ROLE OF THE INTESTINE IN IRON KINETICS. J Clin Invest. 1964 May;43:963–974. doi: 10.1172/JCI104982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu L. L., Fineberg R. A. On the mechanism of iron-induced synthesis of apoferritin in HeLa cells. J Biol Chem. 1969 Jul 25;244(14):3847–3854. [PubMed] [Google Scholar]
- DRYSDALE J. W., MUNRO H. N. SMALL-SCALE ISOLATION OF FERRITIN FOR THE ASSAY OF THE INCORPORATION OF 14C-LABELLED AMINO ACIDS. Biochem J. 1965 Jun;95:851–858. doi: 10.1042/bj0950851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dillard C. J., Tappel A. L. Fluorescent products of lipid peroxidation of mitochondria and microsomes. Lipids. 1971 Oct;6(10):715–721. doi: 10.1007/BF02531296. [DOI] [PubMed] [Google Scholar]
- Drysdale J. W., Munro H. N. Regulation of synthesis and turnover of ferritin in rat liver. J Biol Chem. 1966 Aug 10;241(15):3630–3637. [PubMed] [Google Scholar]
- GOLBERG L., SMITH J. P., MARTIN L. E. The effects of intensive and prolonged administration of iron parenterally in animals. Br J Exp Pathol. 1957 Jun;38(3):297–311. [PMC free article] [PubMed] [Google Scholar]
- Hawkins H. K., Ericsson J. L., Biberfeld P., Trump B. F. Lysosome and phagosome stability in lethal cell injury. Morphologic tracer studies in cell injury due to inhibition of energy metabolism, immune cytolysis and photosensitization. Am J Pathol. 1972 Aug;68(2):255–258. [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
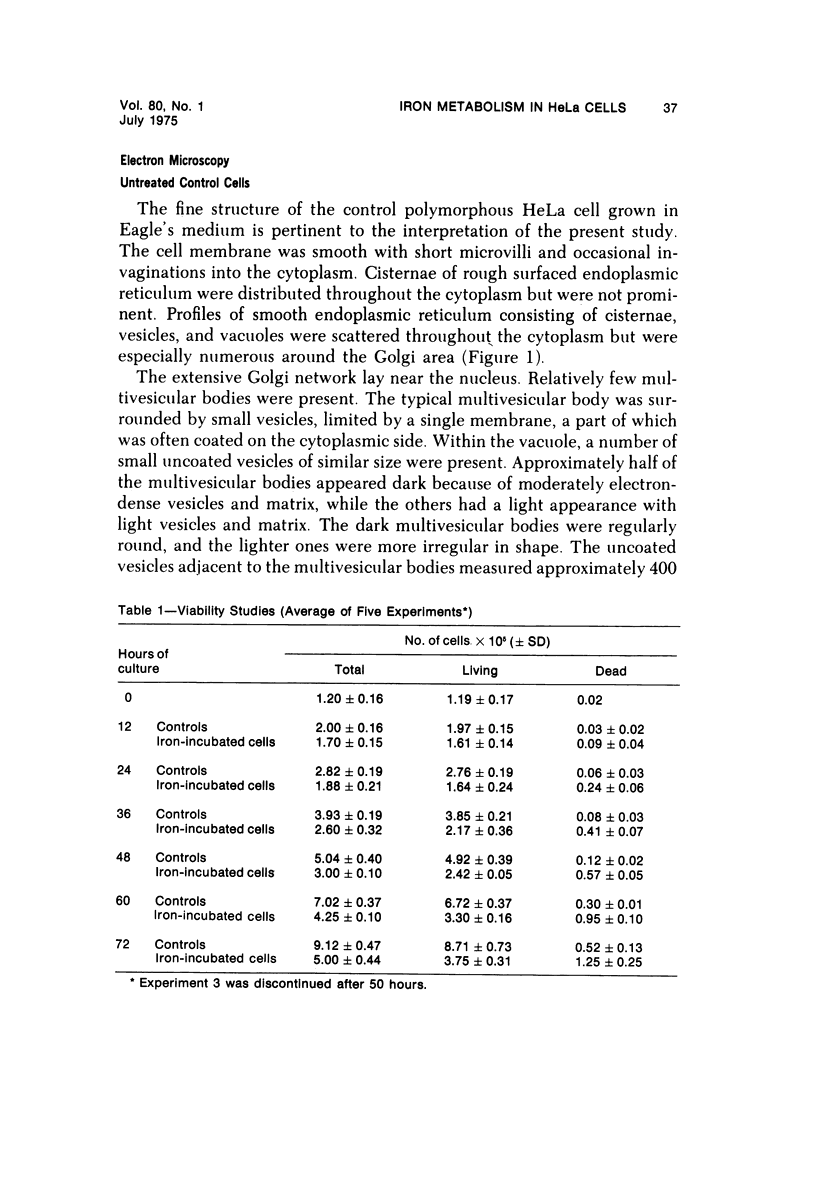
- RICHMOND H. G. The toxic effects of iron-dextran complex on mammalian cells in tissue culture. Br J Cancer. 1961 Sep;15:594–606. doi: 10.1038/bjc.1961.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RICHTER G. W. ON FERRITIN AND ITS PRODUCTION BY CELLS GROWING. Lab Invest. 1963 Oct;12:1026–1039. [PubMed] [Google Scholar]
- Redman C. M. Biosynthesis of serum proteins and ferritin by free and attached ribosomes of rat liver. J Biol Chem. 1969 Aug 25;244(16):4308–4315. [PubMed] [Google Scholar]
- TAPPEL A. L. FREE-RADICAL LIPID PEROXIDATION DAMAGE AND ITS INHIBITION BY VITAMIN E AND SELENIUM. Fed Proc. 1965 Jan-Feb;24:73–78. [PubMed] [Google Scholar]
- Trump B. F., Valigorsky J. M., Arstila A. U., Mergner W. J., Kinney T. D. The relationship of intracellular pathways of iron metabolism to cellular iron overload and the iron storage diseases. Cell sap and cytocavitary network pathways in relation to lysosomal storage and turnover of iron macromolecules. Am J Pathol. 1973 Aug;72(2):295–336. [PMC free article] [PubMed] [Google Scholar]
- Witzleben C. L. An electron microscopic study of ferrous sulfate induced liver damage. Am J Pathol. 1966 Dec;49(6):1053–1067. [PMC free article] [PubMed] [Google Scholar]