Abstract
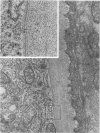
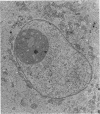
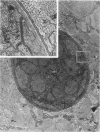
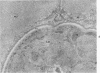
Ultrastructural changes which occurred in infected skeletal muscle fibers after infection with larvae of Trichinella spiralis were followed on a daily basis utilizing synchronous infections. No changes were observed in muscle fiber architecture during the first 2 days of intracellular infection. However, on Day 3, a space containing various sarcoplasmic elements developed between the plasma membrane and myofilaments. Widening near the regions of triads was also observed at this time. On Day 4 the space at the outer edge had increased, as did the ones at the triads. In addition, the myofilaments throughout the infected fiber were in a state of partial disarray. Finally, the nuclei were enlarged and had migrated to the central portion of the infected cytoplasm. On Day 5 day, sarcomeres were highly disorganized, and an increase in sarcoplasmic reticulum (SR) was noted. By Day 8, only the extreme periphery of the infected fiber contained Z bands with actin filaments attached. Proliferation of the t tuble system was also evident. At Day 10, myofilaments were completely replaced with SR. Further, the plasma membrane became hyperinvoluted and was associated with a 36-fold increase in the thickness of the glycocalyx. No further enlargement of nuclei occurred after Day 10. Finally, a host-derived double membrane completely surrounded the larva.
Full text
PDF



















Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Dennis D. T., Despommier D. D., Davis N. Infectivity of the newborn larva of Trichinella spiralis in the rat. J Parasitol. 1970 Oct;56(5):974–977. [PubMed] [Google Scholar]
- Despommier D. A circular thermal migration device for the rapid collection of large numbers of intestinal helminths. J Parasitol. 1973 Oct;59(5):933–935. [PubMed] [Google Scholar]
- HANKES L. V., STONER R. D. Incorporation of DL-tyrosine-2-C-14 and DL-tryptophan-2-C-14 by encysted Trichinella spiralis larvae. Exp Parasitol. 1958 Jan;7(1):92–98. doi: 10.1016/0014-4894(58)90008-0. [DOI] [PubMed] [Google Scholar]
- HUMES A. G., AKERS R. P. Vascular changes in the cheek pouch of the golden hamster during infection with Trichinella spiralis larvae. Anat Rec. 1952 Sep;114(1):103–113. doi: 10.1002/ar.1091140108. [DOI] [PubMed] [Google Scholar]
- Ribas-Mujal D., Rivera-Pomar J. M. Biological significance of the early structural alterations in skeletal muscle fibers infected by Trichinella spiralis. Virchows Arch A Pathol Pathol Anat. 1968;345(2):154–168. doi: 10.1007/BF00548649. [DOI] [PubMed] [Google Scholar]
- STONER R. D., HANKES L. V. Incorporation of C14-labeled amino acids by Trichinella spiralis larvae. Exp Parasitol. 1955 Sep;4(5):435–444. doi: 10.1016/0014-4894(55)90035-7. [DOI] [PubMed] [Google Scholar]
- Stewart G. L., Read C. P. Changes in RNA in mouse trichinosis. J Parasitol. 1973 Dec;59(6):997–1005. [PubMed] [Google Scholar]
- Stewart G. L., Read C. P. Deoxyribonucleic acid metabolism in mouse trichinosis. J Parasitol. 1973 Apr;59(2):264–267. [PubMed] [Google Scholar]
- Stewart G. L., Read C. P. Ribonucleic acid metabolism in mouse trichinosis. J Parasitol. 1972 Apr;58(2):252–256. [PubMed] [Google Scholar]
- Stewart G. L., Read C. P. Some aspects of cyst synthesis in mouse trichinosis. J Parasitol. 1972 Dec;58(6):1061–1064. [PubMed] [Google Scholar]
- Stewart G. L., Read C. P. Studies on biochemical pathology in trichinosis. I. Changes in myoglobin, free creatine, phosphocreatine, and two protein fractions of mouse diaphragm muscle. J Parasitol. 1974 Dec;60(6):996–1000. [PubMed] [Google Scholar]