Abstract
Traumatic brain injury (TBI) is a common cause of cognitive dysfunction and a major risk factor for Alzheimer's disease (AD). PDAPP mice, a transgenic line overexpressing a mutant human amyloid precursor protein (APP) implicated in familial AD, have markedly impaired behavioral performance in the Morris water maze relative to wild-type (WT) littermates. Performance further deteriorates following experimental TBI in both PDAPP and WT mice. However, the aspects of cognitive function involved are not well understood. Here, we have analyzed search strategies used in the water maze by 3–4 month old PDAPP and WT C57Bl6 littermates both before and after moderate controlled cortical impact TBI. Prior to TBI, PDAPP mice used less spatial strategies and more nonspatial systematic strategies and strategies involving repetitive looping than WT mice. With training, PDAPP mice used more spatial strategies and less repetitive looping. After TBI, PDAPP mice lost use of spatial strategies and relied more on repetitive looping. TBI in WT mice also reduced their use of spatial strategies but instead caused a switch to nonspatial systematic strategies. We also analyzed changes in the efficiency with which mice used each individual strategy, but found that differences in which strategies were used quantitatively accounted for most of the differences in performance between groups. These results demonstrate that suboptimal search strategy use in addition to effects on spatial learning and memory underlies the impaired performance of PDAPP mice and further deterioration following TBI. Human TBI patients may have analogous poor use of problem solving strategies.
Keywords: Traumatic brain injury, Alzheimer's disease, Behavior, Morris water maze, Search strategy, Amyloid precursor protein, PDAPP mice
Introduction
Traumatic brain injury (TBI) is a major cause of death and disability, with an estimated incidence of 1.5 million new cases per year in the United States. There are a total of 5.3 million Americans − 2% of the U.S. population –currently living with disabilities resulting from TBI. TBI is also the best-defined environmental risk factor for AD (Mortimer et al., 1991; Plassman et al., 2000; Thurman et al., 1999). Alzheimer's disease (AD) in turn is the principal cause of late-life dementia, affecting approximately 4.5 million people in the United States, with importance increasing as the population ages (Hebert et al., 2003).
Transgenic mice overexpressing human APP are widely used as preclinical models for aspects of AD and TBI (Arendash et al., 2001; Hartman et al., 2002; Irizarry et al., 1997; Murai et al., 1998; Nakagawa et al., 1999; Smith et al., 1998). Behavioral studies of learning and memory, including testing in the Morris water maze, are an important part of these preclinical studies (Crawley, 2000; Fujimoto et al., 2004). PDAPP mice are one such transgenic line that overexpress a mutant human APP that causes a form of autosomal dominant AD (Games et al., 1995). These mice have markedly impaired water maze performance at all ages compared with wild-type littermates (Chen et al., 1998). Furthermore, their performance appears to deteriorate with age and/or development of amyloid-β (Aβ) deposition (Chen et al., 2000). Experimental traumatic brain injury causes further worsening of water maze performance in both wild-type and PDAPP mice (Nakagawa et al., 1999; Smith et al., 1998). However, there is incomplete understanding of the factors influencing water maze performance in these mice.
The Morris water maze is generally considered to be a test of spatial learning and memory (Morris, 1984; Morris et al., 1982). However, this may not always be the case, especially when performance is impaired due to transgene overexpression, drug treatment, and/or brain injury. In these situations, the performance of the mice may depend on other factors such as search strategy use. We found that TBI in PDAPP mice caused a marked deterioration of their performance. However, none of the PDAPP mice –with or without TBI –showed any sign of true spatial memory as assessed using the probe test. The question therefore arose why the brain-injured mice performed so much worse than the uninjured mice. We hypothesized that if two groups of mice have equally poor spatial memory, but one uses more effective search strategies, that this could account for the difference in performance. To test this, we analyzed the predominant search strategy used during each run in the water maze according to a modified version of the schema proposed by Janus (2004). We found, in support of our hypothesis, that the brain-injured mice used less systematic but nonspatial search strategies than the uninjured mice, and instead used more strategies involving repetitive looping, which were much less efficient. Here, we present a detailed analysis of the search strategies used by PDAPP mice both before and after TBI and compare them with those used by their wild-type C57BL6 littermates.
Methods
Mice
We used male and female PDAPP and WT mice from the same litters on a C57Bl6 background starting at 3–4 months of age. The mice were housed in standard cages at 3–5 mice per cage under standard laboratory conditions. All experiments were approved by the animal studies committee at Washington University.
The number of animals analyzed in the pre-TBI and post-TBI data sets is detailed in Table 1. In analyzing the pre-TBI performance data from the mice that either died or were disqualified following TBI, we found significant differences compared with mice that lived and were able to perform the water maze task. Specifically, the performance of the dead/disqualified mice was significantly worse than those that survived and qualified (data not shown). Therefore, the data from the dead/disqualified mice were not included in the pre-TBI analysis in order to be certain that differences between pre-TBI and post-TBI data sets were not due to intrinsic differences between the mice in the two groups. We have also carefully compared the data from the mice that were included in the post-TBI groups presented in this manuscript with those used in the other experiments and found no differences between them. Therefore, in the interest of optimizing statistical power, we have included the data from these mice in the pre-TBI data set. The other experiments involved (1) treatment with a novel therapeutic agent, which was administered starting at the time of the TBI and (2) comparisons of naive mice (no anesthesia, no craniotomy) with sham-injured mice (anesthesia and craniotomy, but no TBI). The results of these experiments are still being analyzed and further experiments are in progress, thus these results are beyond the scope of the current manuscript.
Table 1.
Number of mice analyzed
| PDAPP | WT | |
|---|---|---|
| Initial group | 34 | 35 |
| Died after TBI | 7 | 9 |
| Disqualified from water maze testing after TBI | 4 | 5 |
| Included in pre-TBI data set | 23 | 21 |
| Used in other experiments | 9 | 12 |
| Included in post-TBI data set | 14 | 9 |
Morris water maze testing
In this behavioral test, mice are placed in a pool of water containing a platform just below the surface of the water. They escape from the maze (i.e. they are removed from the pool) when they find the platform. Distal visual cues are arrayed around the room, and in general, mice are able to learn the location of the hidden platform based on these cues.
Because PDAPP mice perform poorly in Morris water maze testing even without TBI, we modified the protocol to facilitate learning as follows: (1) We ran all of the experiments at night, as 3–5 month old PDAPP mice appear to have more pronounced circadian rhythm in body temperature and activity than WT mice (Huitron-Resendiz et al., 2002). (2) The platform was made significantly larger (17.4 cm diameter vs. the typical 10–11 cm) which can improve learning (Crawley, 2000). (3) Each mouse was allowed eight trials per day over an 8–10 h period instead of the usual four trials per day. (4) After arriving on the platform, the mice were allowed to rest 30 s instead of 10. (5) We used very prominent spatial cues including geometric shapes, posters of natural scenes, and a radio tuned to an all talk station. Overall, this protocol resulted in significant improvement over 5 days of training in the PDAPP mice during the first round of testing prior to TBI or sham injury (Fig. 2, P = 0.000002, repeated measures ANOVA). Testing without these modifications resulted in little improvement over 5 days of training (data not shown).
Fig. 2.
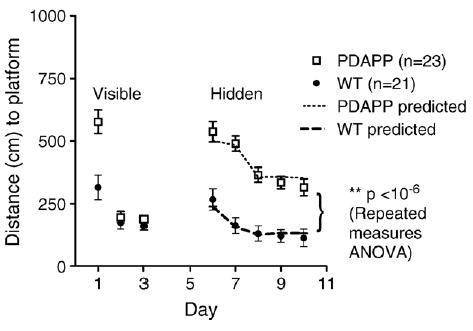
Young PDAPP mice performed considerably worse on the hidden platform portion of the Morris water maze than their wild-type (WT) littermates. Average distance to reach the platform as a function of day of training and genotype. Similar results were seen in an analysis of time to reach the platform. Mice were tested using a modified protocol that facilitated learning in the PDAPP mice: the platform was larger, each mouse was allowed 8 trials per day, all trials were performed at night, and especially prominent spatial cues were used. Dashed lines represent the predicted performance of PDAPP and WT mice based entirely on changes in search strategy use. Dashed lines closely approximate actual performance, indicating that changes in search strategy use largely explain the improvement in performance during the 5 days of hidden platform training.
Visible platform (cued) testing
This portion of the test controls for differences between groups in motivation to escape from the water, swimming ability, and visual acuity. For 3 consecutive days, each mouse was placed in the pool in each of 4 starting locations arrayed around the pool. A clearly visible 17.4 cm diameter plastic platform was placed in one location throughout the 3 days. An automated tracking system (SMART, San Diego Instruments or Polytrack, San Diego Instruments) recorded and analyzed the mouse swim paths. Each trial lasted a maximum of 60 s, and at the end of each trial, the mouse was placed on the platform or allowed to stay on the platform for 30 s. Each mouse was returned to its cage between trials, observed for signs of hypothermia, and warmed with a lamp if necessary. Mice that did not swim to the platform in under 15 s on average by the 3rd day were disqualified and excluded from further testing. No mice were excluded prior to TBI and nine mice were excluded after TBI; there were no differences between groups in terms of number of mice excluded.
Hidden platform (place) testing
This portion of the test assesses the ability of the mice to find the platform under conditions where they cannot directly see it, but must either remember where it is relative to external cues or perform a search for it. The platform was placed 1 cm under the surface of the water, and the water was made opaque by a suspension of white, nontoxic tempera paint. The platform was placed in a different location from that used in visible platform testing. Each mouse was released from one of 4 locations and had 60 s to search for the hidden platform. At the end of each trial, the mouse was placed on the platform or allowed to stay on the platform for 30 s. Prominent spatial cues were arrayed around the room. The investigator is also a powerful spatial cue and always sat in the same location during each trial after releasing the mouse. Eight trials per day for 5 consecutive days were performed with the location of the platform kept constant.
Probe trial
The day after the completion of hidden platform testing, the platform was removed, and each mouse was placed in the pool once for 30 s, starting from the same starting location as was used first in hidden platform testing. The time spent swimming in the quadrant where the platform had been was recorded. This is considered to be the most specific test for spatial memory (Crawley, 2000).
Search strategy analysis
The swim path for each trial (each time a mouse was placed in the pool) during visible and hidden platform testing was plotted using either the SMART system (San Diego Instruments) or the coords.exe utility from the Polytrack system (San Diego Instruments) along with a custom written Matlab routine. A single investigator (DLB) blinded to mouse genotype and injury status assigned a predominant search strategy to each trial using a categorization scheme similar those developed previously (Graziano et al., 2003; Janus, 2004; Lang et al., 2003; Wolfer and Lipp, 2000). Mice occasionally appeared to switch strategies during a trial. When this occurred, the strategy that best described the majority of the swim path was assigned. The categorization scheme was modified iteratively to fit the actual behaviors observed in PDAPP mice. In reanalysis of a subset of trials by the same investigator 1 week later, intraobserver agreement on strategy classification was 97%. Manual categorization of search strategy was not overly time consuming; 3169 trials were analyzed in less than 1 week. In principle, strategy analysis could potentially be performed while the data are being acquired.
Spatial strategies (Fig. 1A) included 'spatial direct' (swimming directly to the platform), 'spatial indirect' (swimming to the platform with at most one loop), and 'focal: correct target quadrant' (swimming directly to and searching intently in the quadrant containing the platform).
Fig. 1.
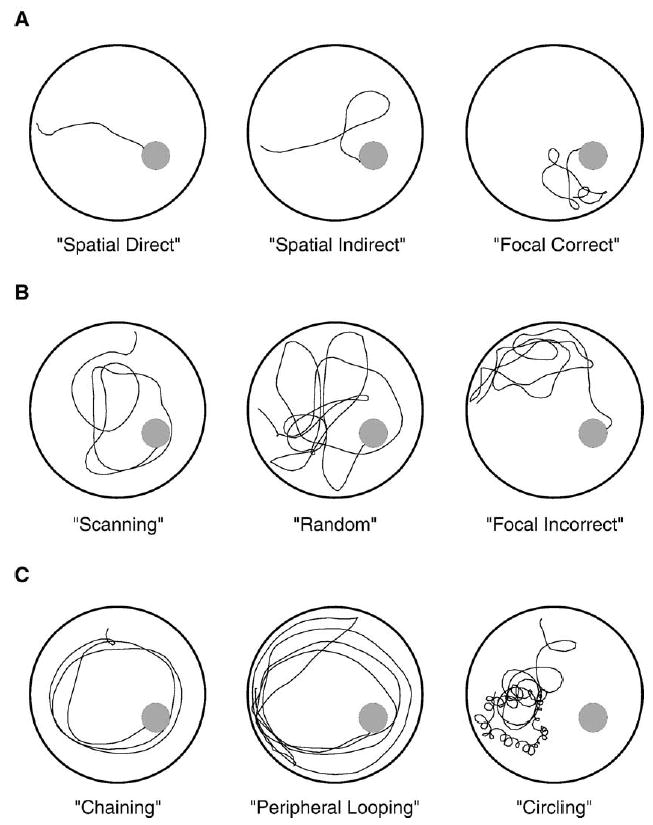
Examples of search strategies used by young PDAPP mice in the hidden platform portion of the Morris water maze. The location of the hidden platform is indicated by the filled gray circle. (A) Spatial strategies. (B) Nonspatial, systematic strategies. (C) Strategies based on repetitive looping. Please see Methods for operational definitions of the individual strategies. Note that the 'chaining' performed by PDAPP mice was considerably less efficient than the sort of chaining that has been described in other transgenic mice, and therefore was grouped with the repetitive looping strategies.
Systematic but nonspatial strategies (Fig. 1B) included 'scanning' (searching the interior portion of the tank without spatial bias), 'random' (searching the entire tank without bias towards any portion), and 'focal: incorrect target quadrant' (searching intently a small portion of the tank that does not contain the platform, then possibly moving to another area of the tank).
Strategies involving repetitive looping paths (Fig. 1C) included 'chaining' (circular swimming at an approximately fixed distance greater than 15 cm from the wall). 'peripheral looping' (persistent swimming around the outer 15 cm of the pool), and 'circling' (swimming in tight circles, possibly with some net directional movement). True 'thigmotaxis' or wall-hugging behavior was rare in these experiments, but when it occurred, it was grouped with peripheral looping. Of note, the 'chaining' paths taken by PDAPP mice differed from those described for adenosine receptor knockout (Lang et al., 2003) and CNRD8 mice (Janus, 2004); the paths were at an approximately fixed distance from the wall, but often it was not within the range of distances that would result in efficient arrival at the platform. Thus, this 'inefficient chaining' was similar to other repetitive looping strategies. Rarely, mice were best described as 'floating' (remaining motionless most of the time); these trials were excluded.
Statistical methods
All data were analyzed using Statistica 6.0 (StatSoft). For Morris water maze performance data, repeated measures ANO-VAs were used. For probe trial data, 95% confidence intervals were calculated and compared to performance expected by chance. For comparisons of strategy use between groups, chi-square analysis was performed. Significance was defined as P < 0.05 after Bonferroni correction for multiple comparisons.
Traumatic brain injury
Mice underwent a single, moderate left lateral controlled cortical impact with craniotomy, as described previously (Dixon et al., 1991; Murai et al., 1998; Smith et al., 1998; Smith et al., 1995). This procedure results in a near complete destruction of the underlying frontoparietal cortex and substantial cell loss in the ipsilateral hippocampus. Contralateral structures are undamaged.
Mice were anesthetized i.p. with 65 mg/kg pentobarbital. Ten minutes later, ointment to protect vision was applied to their eyes, and they were placed in a stereotactic frame on a warming pad. The top of the skull was exposed and a 5 mm craniotomy was performed over the left frontoparietal cortex using a hand trephine. Care was taken not to penetrate the dura during this procedure. 45 minutes after anesthesia, animals were subjected to controlled cortical impact (CCI) in which a 3 mm flat metal tip impounder was driven by a pneumatic cylinder at a velocity of 5 m/s to a depth of 1 mm into the cortex. Sham-treated animals were anesthetized, had a craniotomy, and were placed in the CCI device but did not undergo CCI TBI. Sham injury does not affect behavioral performance or cause detectable tissue loss relative to naive mice (data not shown). Mice were removed from the CCI device, the stereotactic frame detached, and a plastic skull cap glued to the skull with veterinary adhesive to cover the craniotomy site. The skin was then closed with interrupted 4–0 silk sutures and mice were allowed to recover on a warming pad. They were returned to their home cages when fully ambulatory, usually within 1.5 h after induction of anesthesia.
Results
Search strategy use in PDAPP and wild-type mice
On the first day of visible platform (cued) testing prior to TBI, PDAPP mice performed worse than WT mice but on the 2nd and 3rd days, the PDAPP mice performed as well as the wild-type mice (P = 0.23, repeated measures ANOVA). Overall, this indicated that the PDAPP mice did not appear to differ in visual function, swimming ability, and motivation to escape from the pool (Fig. 2). On the first day of hidden platform testing prior to TBI, PDAPP mice had poor overall performance compared with wild-type littermates. The performance of both PDAPP and WT littermates improved over the 5 days of training (P < 10−6, repeated measures ANOVA), but the performance of the PDAPP mice was always significantly worse than that of WT mice (P < 10−6).
PDAPP mice used a mixture of strategies on the first day of hidden platform testing (Fig. 3A) including spatial, nonspatial, systematic, and a relatively high proportion (23%) of repetitive looping-based strategies. Over the subsequent 4 days, the fraction of repetitive looping-based strategies declined to under 8% by the 5th day (P < 0.0001, chi-square). The fraction of true spatial strategies increased during this time from 52% on day 1 to 66% on day 5 (P = 0.0081). Systematic nonspatial strategy use stayed relatively constant at 24% to 26% (P = 0.72). Despite some evidence of spatial strategy use, the performance of these mice on the probe test was barely above chance. They spent only 32% of the time in the target quadrant (95% confidence interval 27%–37%) and 5.1% in the exact area where the platform had been (95% confidence interval 3.3%–6.9%). The expected percentage due to chance was 25% for the target quadrant and 3% for the exact area where the platform had been, as the platform's area was approximately 3% of the total tank's area. Thus, the PDAPP mice used spatial strategies frequently during hidden platform testing but did not seem to have developed robust spatial memory, defined as probe trial performance markedly above that which would be expected due to chance.
Fig. 3.
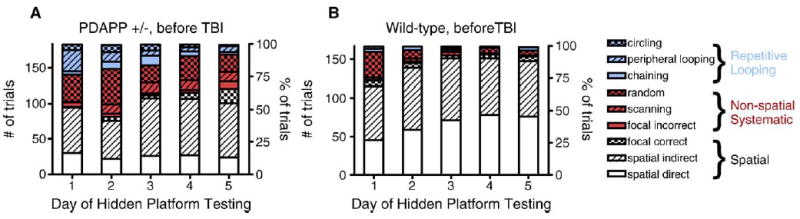
Search strategy use differed markedly between PDAPP and WT mice, and strategy use changed over the 5 days of hidden platform testing. Number of trials (left axis) and percentage of trials (right axis) using a given strategy plotted as a function of genotype and day of hidden platform training. (A) PDAPP mice. During the 5 days of training in PDAPP mice, repetitive looping strategy use decreased from 23% on day 1 to 8% on day 5 (P < 0.0001, chi-square), systematic nonspatial strategy use stayed relatively constant at 24% to 26% (P = 0.72), and spatial strategy use increased from 52% to 66% (P = 0.0081). (B) WT mice. WT mice used repetitive looping very infrequently but decreased their use of nonspatial systematic strategy use from 23% to 5% (P < 0.0001) and increased their use of spatial strategies from 73% to 92% (P < 0.0001). PDAPP mice used fewer spatial and more nonspatial, systematic and repetitive looping strategies than WT mice (P < 0.0001, chi-square for each comparison).
In contrast, even on the first day of hidden platform testing, young wild-type C57Bl6 mice used a high proportion (75%) of spatial strategies along with some nonspatial systematic strategies (23%) and very few repetitive looping strategies (2%, Fig. 3B). As their performance improved, they used spatial strategies nearly exclusively. The performance of the wild-type mice on the probe test was well above chance; 51% of their time was spent in the target quadrant (95% confidence interval 47%–55%) and 14.4% of their time was spent in the exact area where the platform had been (95% confidence interval 11.9%–16.9%). This indicated robust spatial learning and memory.
Quantitative analysis of the contribution of search strategy to overall performance
As the improvement in performance over the 5 days of hidden platform testing in the PDAPP mice appeared to be associated only with weak spatial memory (as assessed by the probe trial), we next asked whether the shift in search strategy use was responsible for the improved performance. An alternative possibility was that the mice got better at using one or more of the individual strategies over the 5 days of training and that this resulted in their improved performance. To determine whether this alternative hypothesis was tenable, we calculated the average swim distance associated with each individual strategy on the 1st day of testing and again on the 5th day of testing (Table 2). We found statistically significant decreases from day 1 to day 5 in the swim distances associated with repetitive looping (P < 0.0001 for PDAPP and P < 0.01 for WT mice) and nonspatial systematic strategies (P < 0.0001 for PDAPP and P < 0.0001 for WT mice), whereas the distances associated with spatial strategies were stable during training for PDAPP mice and improved for WT mice (P < 0.0001).
Table 2.
Performance as a function of strategy use in hidden platform Morris water maze testing
| Strategy | PDAPP day 1 | PDAPP day 5 | PDAPP after sham | PDAPP after TBI | WT day 1 | WT day 5 | WT after sham | WT after TBI |
|---|---|---|---|---|---|---|---|---|
| Repetitive looping | 1152 ± 61
n = 43 |
941 ± 108
n = 14 |
467 ± 59
n = 27 |
859 ± 35
n = 78 |
256 ± 54
n = 4 |
137 ± 48
n = 5 |
89
n = 1 |
401
n = 1 |
| Circling | 1124 ± 73
n = 8 |
476 ± 146
n = 2 |
664 ± 71
n = 5 |
816 ± 112
n = 2 |
N/A | N/A | N/A | 401
n = 1 |
| Peripheral looping | 1293 ± 38
n = 30 |
1386 ± 73
n = 8 |
1354
n = 1 |
1122 ± 27
n = 35 |
N/A | N/A | N/A | N/A |
| Chaining | 354 ± 93
n = 5 |
286 ± 104
n = 4 |
378 ± 35
n = 21 |
636 ± 25
n = 41 |
256 ± 54
n = 4 |
137 ± 48
n = 5 |
89
n = 1 |
N/A |
| Non-spatial, systematic | 747 ± 47
n = 45 |
513 ± 45
n = 48 |
443 ± 26
n = 97 |
668 ± 36
n = 49 |
626 ± 44
n = 37 |
425 ± 96
n = 8 |
450 ± 49
n = 27 |
519 ± 33
n = 61 |
| Random | 824 ± 34
n = 38 |
740 ± 42
n = 24 |
477 ± 28
n = 33 |
710 ± 25
n = 40 |
646 ± 18
n = 34 |
457 ± 44
n = 6 |
552 ± 48
n = 11 |
606 ± 25
n = 41 |
| Scanning | 473
n = 1 |
267 ± 55
n = 14 |
404 ± 21
n = 58 |
523 ± 56
n = 8 |
388 ± 62
n = 3 |
326 ± 76
n = 2 |
364 ± 44
n = 13 |
310 ± 41
n = 15 |
| Focal incorrect | 305 ± 85
n = 6 |
315 ± 66
n = 10 |
462 ± 65
n = 6 |
159
n = 1 |
N/A | N/A | 443 ± 91
n = 3 |
293 ± 71
n = 5 |
| Spatial | 162 ± 10
n = 96 |
166 ± 9
n = 121 |
108 ± 7
n = 132 |
130 ± 9
n = 65 |
143 ± 7
n = 122 |
95 ± 7
n = 153 |
91 ± 7
n = 132 |
145 ± 9
n = 66 |
| Focal correct | 1067
n = 1 |
343 ± 46
n = 20 |
197 ± 56
n = 8 |
298 ± 112
n = 2 |
355 ± 40
n = 7 |
198 ± 44
n = 6 |
214 ± 65
n = 6 |
319 ± 46
n = 12 |
| Spatial indirect | 198 ± 26
n = 65 |
149 ± 24
n = 77 |
140 ± 18
n = 77 |
163 ± 24
n = 43 |
155 ± 13
n = 70 |
116 ± 13
n = 71 |
135 ± 22
n = 52 |
128 ± 25
n = 39 |
| Spatial direct | 54 ± 38
n = 30 |
73 ± 42
n = 24 |
43 ± 23
n = 47 |
43 ± 35
n = 20 |
92 ± 16
n = 45 |
68 ± 12
n = 76 |
50 ± 18
n = 74 |
48 ± 41
n = 15 |
Distance in cm (mean ± standard error). n: number of individual trials during hidden platform testing where a given strategy was used. N/A: not applicable; no examples of this search strategy were found. Shaded rows: classes of strategies. Values reflect the pooled results from the 3 specific strategies listed below each shaded row.
Therefore, we proceeded to assess the importance of the shift in strategies relative to the improved efficiency when using each strategy. To do this, we performed a convolution analysis (Yue et al., 1990); the average performance across all 5 days given each particular strategy was multiplied by the frequency with which each strategy was used on each day. For example, the average swim distance across all 5 days of pre-TBI testing when PDAPP mice used the spatial direct strategy was 61 cm, spatial indirect: 167 cm, focal correct: 429 cm, focal incorrect: 336 cm, scanning: 471 cm, random: 744 cm, chaining: 405 cm, peripheral looping: 1311 cm, and circling: 840 cm. On day 1, PDAPP mice used the spatial direct strategy 16% of the time, spatial indirect: 35%, focal correct: 1%, focal incorrect: 3%, scanning: 1%, random: 21%, chaining: 3%, peripheral looping: 16% cm, and circling: 4%. The predicted performance using convolution analysis therefore was:
61 × 0.016 + 167 × 0.35 + 429 × 0.01 + 336 × 0.03 + 471
× 0.01 + 744 × 0.21 + 405 × 0.03 + 1311 × 0.16 + 840
× 0.04 = 499 cm.
The actual performance was 537 ± 41 cm. The same sort of analysis applied to the search strategy use of the PDAPP mice on day 5 yielded a predicted performance of:
61 × 0.13 + 167 × 0.42 + 429 × 0.11 + 336 × 0.05 + 471
× 0.08 + 744 × 0.13 + 405 × 0.02 + 1311 × 0.04 + 840
× 0.01 = 353 cm.
Thus, this analysis removes the changes in performance given a particular strategy from the assessment and leaves only the shift in strategy use as the determinant of predicted performance. The predicted performance based on the convolution analysis matched the actual measured performance closely, though not exactly (Fig. 2, dashed lines); this analysis predicted a 29% improvement in performance from day 1 to day 5, as compared to the 41% improvement that was observed in the actual data.
We next performed the converse analysis, in which we held constant the frequency with which each strategy was used and examined the effects of the performance improvements within each strategy in isolation. This revealed a predicted improvement in performance by 9.6% between day 1 and day 5. Thus, we conclude that shifts in search strategy use were primarily responsible for the improved performance during the 5 days of training in PDAPP mice, but that improved performance using the repetitive looping and nonspatial systematic strategies also contributed.
A similar analysis was applied to the WT mice. The convolution analysis predicted a 45% improvement over the 5 days of training due to shifts in search strategy use, whereas the observed improvement was 58% (Fig. 2, dashed lines). The performance improvements within each strategy predicted a 29% improvement. Thus, in wild-type mice, shifts in search strategy use still played the predominant role in the improved performance during training, but improvements in the use of each individual strategy may have contributed more substantially than in PDAPP mice.
Next, we asked whether the changes in search strategy use explained the differences in performance between PDAPP and WT mice. We analyzed the average swim distance across all 5 days of training associated with each of the strategies used by both genotypes (some strategies used by PDAPP mice were never observed in WT mice, see Table 2). The strategy choices made by WT mice predicted a 41% better performance compared with the strategy choices made by PDAPP mice when performance given the strategy chosen was held constant at the mean value for the PDAPP mice. The observed improvement from the actual data was 61%. Overall, WT mice had better performance within several strategies compared with PDAPP mice (Table 2). The performance improvements within each strategy yielded an 18% improvement in total performance when search strategy use was held constant. Thus, changes in which search strategies were used appeared to quantitatively explain most of the difference in performance between PDAPP and WT mice.
We found in general, that most individual mice used a mixture of strategies over the 8 trials per day of testing (Table 3). Overall, the analysis of how many mice used each individual strategy was much less sensitive than the analysis of number of trials during which each strategy was used. For example, while it was apparent that PDAPP mice used more nonspatial, systematic strategies and less spatial strategies than WT mice, the analysis of individual mice revealed that at some point, every PDAPP mouse and every WT mice used both nonspatial, systematic strategies and spatial strategies.
Table 3.
Number of mice that used each search strategy (clear rows) or group of search strategies (shaded rows) at any point during testing
| Strategy | PDAPP before TBI | PDAPP after sham | PDAPP after TBI | WT before TBI | WT after sham | WT after TBI |
|---|---|---|---|---|---|---|
| Repetitive looping | 23/23 (100%) | 7/8 (88%) | 6/6 (100%) | 14/21 (67%) | 1/5 (20%) | 1/4 (25%) |
| Circling | 5/23 (22%) | 1/8 (13%) | 1/6 (17%) | 0/21 (0%) | 0/5 (0%) | 1/4(25%) |
| Peripheral looping | 18/23 (78%) | 1/8 (13%) | 6/6 (100%) | 3/21 (14%) | 0/5 (0%) | 0/4 (0%) |
| Chaining | 17/23 (74%) | 7/8 (13%) | 6/6 (100%) | 13/21 (62%) | 1/5 (20%) | 0/4 (0%) |
| Non-spatial systematic | 23/23 (100%) | 8/8 (100%) | 6/6 (100%) | 21/21 (100%) | 4/5 (80%) | 4/4(100%) |
| Random | 23/23 (100%) | 8/8 (100%) | 6/6 (100%) | 21/21 (100%) | 4/5 (80%) | 4/4(100%) |
| Scanning | 18/23 (78%) | 8/8 (100%) | 4/6 (67%) | 12/21 (57%) | 3/5 (60%) | 4/4(100%) |
| Focal incorrect | 16/23 (70%) | 4/8 (50%) | 1/6 (17%) | 3/21 (14%) | 2/5 (40%) | 4/4 (100%) |
| Spatial | 23/23 (100%) | 8/8 (100%) | 6/6 (100%) | 21/21 (100%) | 5/5(100%) | 4/4(100%) |
| Focal correct | 17/23 (74%) | 4/8 (50%) | 1/6 (17%) | 15/21 (71%) | 4/5 (80%) | 4/4(100%) |
| Spatial indirect | 23/23 (100%) | 8/8 (100%) | 6/6 (100%) | 21/21 (100%) | 5/5(100%) | 4/4(100%,) |
| Spatial direct | 22/23 (96%) | 8/8 (100%) | 6/6 (100%) | 21/21 (100%) | 5/5(100%) | 4/4(100%) |
Values for groups of search strategies may be larger than the sum of the values for individual search strategies within the group because each mouse may use more than one of the search strategies within each group.
Search strategy use following experimental traumatic brain injury
One week after completion of water maze testing, mice were subjected to either experimental controlled cortical impact traumatic brain injury (TBI) or sham injury, which consisted of pentobarbital anesthesia and a craniotomy, but no actual brain injury. Two weeks later, they were tested again in the water maze in a different room with different spatial cues and with the platform placed in a different location. Overall, both PDAPP mice and WT mice had impaired performance following TBI compared to sham-injured mice of the same genotype (Fig. 4) (P < 0.00001, Repeated Measures ANOVA), and PDAPP mice performed worse than WT mice following TBI (P < 0.00001, repeated measures ANOVA).
Fig. 4.
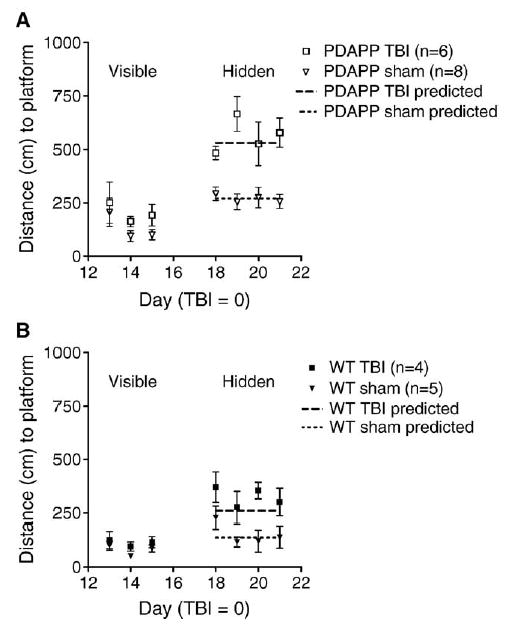
Performance of both PDAPP and WT mice worsened following experimental controlled cortical impact TBI (P < 0.00001, repeated measures ANOVA). Mice were retested in a different room with the platform in a different location and different spatial cues. Sham-treated mice received anesthesia and a craniotomy but no TBI. Assessments were made blinded to genotype and injury status. Changes in search strategy use largely explain the impaired performance following TBI. Dashed lines represent the predicted performance of PDAPP and WT mice with and without TBI based entirely on search strategy use; the efficacy with which the mice used each of the individual search strategies was held constant. Data from all 4 days of post-TBI hidden platform training were combined for this analysis. (A) PDAPP mice. (B) WT mice.
However, there were marked differences between the genotypes in terms of how TBI affected strategy use (Fig. 5). Wild-type mice significantly reduced their use of spatial strategies (P < 0.0001, chi-square), mainly the spatial direct strategy, and increased their use of nonspatial, systematic strategies (P < 0.0001, chi-square) such as random searching. Qualifying WT mice used very few repetitive looping strategies even after TBI. In contrast, PDAPP mice significantly reduced their use of both spatial (P = 0.0002, chi-square) and nonspatial systematic (P = 0.0057, chi-square) strategies, scanning in particular, and increased their reliance on repetitive looping strategies (P < 0.0001, chi-square). These conclusions were based on combined analysis from all 4 days of hidden platform testing as there was little change in performance across days in any of the injured or sham-injured animals (Fig. 4).
Fig. 5.
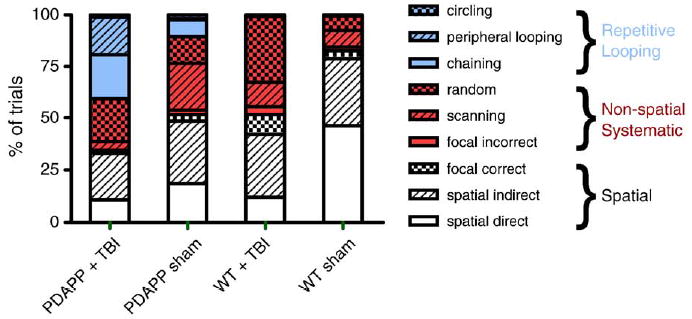
Search strategy use was markedly affected by TBI in both PDAPP and WT mice, but the two genotypes responded differently. Percentage of trials using each strategy as a function of genotype and injury status. PDAPP mice subjected to TBI used more repetitive looping strategies (P < 0.0001) and less spatial (P = 0.0002) and nonspatial systematic strategies (P < 0.017) than uninjured PDAPP mice. In contrast, WT mice subjected to TBI used more nonspatial systematic strategies (P < 0.0001) and less spatial strategies (P < 0.0001) than uninjured WT mice. Data from all 4 days of post-TBI hidden platform training were combined for this analysis.
We found highly statistically significant differences between PDAPP and WT mice despite the relatively small numbers of mice. In terms of search strategy use, PDAPP mice used significantly less spatial strategies after TBI than WT mice after TBI (P = 0.0016, chi-square), significantly less systematic, nonspatial strategies (P < 0.0001, chi-square), and significantly more repetitive looping strategies (P < 0.0001, chi-square).
For the most part, WT mice were just as good at finding the platform when they used each individual strategy after TBI as before injury or after sham injury. PDAPP mice instead were markedly worse at using the random and chaining strategies after TBI than before injury or after sham injury (Table 2). Once again, we analyzed the extent to which these shifts in strategy use contributed to overall performance as compared to changes in the ability of the mice to perform each individual strategy. We were able to predict the performance of the brain-injured mice accurately using convolution analysis, taking the performance given each individual strategy from the pre-TBI data and multiplying by the fraction of strategy use after TBI (Fig. 4, dashed lines). Specifically, the shifts in strategy use predicted a 96% increase in swim distance following TBI in the PDAPP mice whereas the observed impairment was 109%, averaging over all 4 days of hidden platform testing. The predicted impairment was 87% following TBI in the WT mice, whereas the observed impairment was 115%.
The converse analysis, holding strategy use constant and examining the effects of impairments in the performance within a given strategy, predicted an impairment of 31% in the PDAPP mice and less than 1% in the WT mice. Again, changes in search strategy use explained most of the impaired performance following TBI.
Next, we analyzed whether there were signs of excessive fatigue or stress on the mice during the 8 trials per day. We found no differences in swim speeds between the first and last trials in each day during the 5 days of hidden platform testing prior to TBI. However, in the 4 days of hidden platform testing after TBI, there was an approximately 12% decrease in velocities from trial 1 (19.7 cm/s) to trial 8 (17.3 cm/s) which was statistically significant (P = 0.003). This effect occurred on each of the 4 days of testing and was similar in all 4 experimental groups (P = 0.81, run number * group interaction, repeated measures ANOVA). However, overall performance (distance to the platform) actually improved by 11% from trial 1 to trial 8 each day following TBI. Following TBI, use of spatial strategies increased from 39% to 52% (P = 0.012, chi-square) when comparing the first 2 runs of each day with the last 2 runs. This was most marked in the WT mice subjected to TBI; these mice increased use of spatial strategies from 33% to 65% (P = 0.001, chi-square), WT mice also decreased use of systematic, nonspatial strategies from 66% down to 31% (P = 0.0005). This further indicates that search strategy analysis may improve our ability to interpret water maze data in that it demonstrates that a decrease in swim velocity may not necessarily indicate fatigue or stress, but rather a shift in search strategy.
During visible platform testing, both PDAPP and WT mice subjected to TBI still used a high proportion (>70%) of spatial strategies. There were no statistically significant differences between PDAPP and WT mice with or without TBI in search strategy use during visible platform testing (P > 0.05, chi-square, data not shown). This was in accordance with our finding that overall visible platform performance was normal in PDAPP mice, and in mice of both genotypes subjected to TBI (Fig. 4). However, this analysis included only those mice that qualified by performing the visible platform task in under 15 s by the 3rd day of visible platform testing. Analysis of disqualified mice revealed a marked impairment in strategy use; disqualified mice used spatial strategies only 43% of the time whereas 25% of the trials were categorized as 'circling' and 7% as 'floating. ' There were no differences between groups in terms of number of mice excluded. In qualified mice, floating and circling behavior occurred less that 1% of the time during visible platform testing. Many of the disqualified mice had motor impairments which likely accounted for their poor performance, whereas circling and floating in qualified mice during hidden platform testing did not appear to reflect a motor deficit, as by definition they performed well during visible platform testing and neither circling nor floating ever resulted in arrival at the platform in under 15 s.
Discussion
In summary, we have found that young PDAPP mice use a qualitatively and quantitatively different set of search strategies than their wild-type littermates in the Morris water maze. In particular, PDAPP mice used fewer spatial strategies and more nonspatial systematic strategies as well as more repetitive looping-based strategies. In both groups, the use of search strategies changed over several days of training, and these changes were the primary cause of their improved performance. In both groups, search strategy selection was dramatically altered following experimental traumatic brain injury and again these changes were primarily responsible for their impaired performance.
There was no evidence of Aβ deposition in these young PDAPP mice with or without TBI (data not shown). It is notable from our study and the work of others (Chen et al., 2000; Chen et al., 1998; Dodart et al., 1999, 2000, 2002; Smith et al., 1998) that the impaired behavioral performance of PDAPP mice is at least partially dissociated from Aβ deposition. These mice are abnormal behaviorally as early as they can be tested, but Aβ deposition does not begin until at least 6 months of age. In addition, passive vaccination with anti-Aβ antibodies improves behavioral performance acutely, in the absence of short-term effects on Aβ deposition (Dodart et al., 2002; Kotilinek et al., 2002). These findings taken together suggest that elevated levels of a toxic, soluble Aβ species may be responsible for at least some of the behavioral deficits in APP transgenic mice. Aβ levels have been shown to rise acutely after TBI in young PDAPP mice (Smith et al., 1998) and this could potentially contribute to the cognitive dysfunction that follows TBI.
Search strategy analysis helped explain many aspects of the performance of PDAPP mice that would otherwise have been difficult to interpret. First, the performance of these mice improved over 5 days of hidden platform testing, but they showed minimal evidence of true spatial memory in the probe test. We found that one of the main effects of training was reducing the number of repetitive looping strategies used and increasing the use of more efficient nonspatial systematic strategies. This appears to reflect procedural learning. Second, when the PDAPP mice were retested in the water maze with different distal cues and with the platform in a different position, their initial hidden platform performance was as good as it had been at the end of 5 days of training in the previous maze (compare day 10 in Fig. 2 with day 18 in Fig. 4). This appeared to be due to retained patterns of search strategy use, which we interpret as reflecting procedural memory. Third, following experimental TBI, hidden platform performance worsened and did not significantly improve with 4 days of intensive training. The injured PDAPP mice returned to increased reliance on repetitive looping strategies. Our interpretation is that their procedural memory was disrupted by the TBI, and the mice appeared to have lost the capacity for procedural relearning.
We found a pronounced difference between the effects of TBI on strategy use in PDAPP mice and WT mice. WT mice reduced use of spatial strategies and increased use of nonspatial, systematic strategies. Instead, PDAPP mice decreased use of both spatial and nonspatial, systematic strategies, and increased use of repetitive looping strategies. The reason for this genotype difference is not known; however, we hypothesize that elevated levels of human Aβ following TBI in PDAPP mice (Smith et al., 1998) (which do not occur in WT mice) may interfere with strategy use. Further experiments involving manipulation of Aβ levels at the time of TBI will be necessary to resolve this issue.
Search strategy analysis has been used to analyze the performance of rats and transgenic mice in the past (Graziano et al., 2003; Grootendorst et al., 2001; Janus, 2004; Lang et al., 2003; Sutherland et al., 1982; Wolfer and Lipp, 2000). The current study is novel in that it represents the first quantitative dissection of the contribution made by changes in strategy use as compared with changes in performance using each individual strategy. The overall finding was that both strategy choice and ability to use each strategy contributed to overall performance, but that strategy choice played a predominant role. This may lead to greater focus in the future on procedural aspects of the water maze task, independently of and interacting with assessments of spatial learning and memory.
Our results reveal that search strategy use is more complex than has been suggested in the past (Sutherland et al., 1982). A simple hierarchical hypothesis that mice capable of using spatial information should use spatial strategies nearly 100% of the time, whereas those incapable of using spatial information should use predominantly nonspatial, systematic strategies proved incorrect in the present study. Instead, even PDAPP mice and WT mice subjected to TBI with little evidence of true spatial memory in the probe task used spatial strategies in a substantial number of trials. In some respects, this is similar to rats subjected to medial caudate–putamen lesions that can use spatial strategies, but prefer alternative, possibly simpler strategies (Whishaw et al., 1987).
In cases where it may be difficult to determine whether an effect on water maze performance is due to cognitive or non-cognitive (motor, sensory) function, a quantitative analysis of search strategy use may be helpful. In a closely related study, we found that administration of an anti-Aβ antibody to PDAPP mice improved their water maze performance in terms of distance following TBI. However, effects of antibody treatment on latency were not significant. Analysis of search strategies revealed that the antibody-treated mice used significantly fewer repetitive looping strategies compared to untreated mice, and that the lack of a significant effect on latency occurred because the velocities associated with repetitive looping strategies were greater than those associated with systematic, nonspatial strategies (manuscript in preparation).
An important point to emphasize is that the specific strategy analysis may need to be individualized for each experimental situation. For example, chaining plays a relatively minor role in uninjured PDAPP mice, whereas it is appears to be a central part of the repertoire of TgCRND8 mice (Janus, 2004). In addition, the contribution to overall performance of changes in search strategy must be assessed quantitatively. For example, it is not clear whether the development of chaining in TgCRND8 mice truly underlies their improved performance during training, as chaining use increased dramatically on days 2 and 3 of training, whereas overall performance did not improve until day 4. As rapid arrival at the platform using chaining relies on an accurate estimation of the distance between the wall and the location of the hidden platform, it is possible that mice may improve their chaining performance with training. We observed significantly better chaining performance in uninjured PDAPP mice than in PDAPP mice subjected to TBI. When PDAPP mice subjected to TBI did use chaining, it appeared to be very inefficient; they swam around the pool at a fixed distance from the wall, but the distance was often either too close or too far from the wall to result in rapid arrival at the hidden platform.
In the future, to further investigate the effects of TBI on procedural learning, it will be important to test mice subjected to TBI that had not been previously exposed to the water maze. The distinction between procedural learning and spatial learning could be made even more directly by using a nonspatial pretraining paradigm (Morris, 1989; Saber and Cain, 2003) in which the mice are first trained to perform the task with the location of the hidden platform changed between each trial.
The physiological basis for differences in search strategy between animals is unknown. Muscarinic blockade with atropine has been shown not to affect performance in a nonspatial version (visible platform) of the Morris water maze, but alters the strategies used in initially searching for the platform in a subsequent spatial version (hidden platform) (Whishaw, 1989; Whishaw and Petrie, 1988). This suggests that integrity of the cholinergic system may play a role in search strategy selection. In our experiments, both APP transgene overexpression and experimental TBI likely have pleiotropic effects on the central nervous system, so further work will be needed to clarify the structural and neurochemical mechanisms involved.
A recent report suggested that phosphorylated CREB immunoreactivity (pCREB-IR) was increased in the hippocampus in rats that used an allocentric, spatial strategy to solve a radial arm maze task, whereas pCREB-IR was increased in the striatum of rats that used an egocentric, response strategy (Colombo et al., 2003). Strategy choice was determined for each rat based on a single probe trial and immunoreactivity was measured immediately afterwards. A question that therefore arises is whether these changes reflected an intrinsic difference between the animals, or were a response to the specific choice that the rats made during the probe trial. It will be interesting in the future to analyze the search strategy patterns of individual mice subjected to TBI and correlate these with changes in pCREB-IR.
The clinical relevance of these analyses is underscored by an emerging literature on cognitive strategy use in humans who have suffered brain injuries. In one study, a group of chronic TBI patients with cognitive deficits and reduced expressive language function was trained to use a computer-based augmentative communication device (Burke et al., 2004). They were asked to select the same group of words organized using three different strategies: alphabetical, by associated place (kitchen, doctor's office, mall, etc.) or by associated topic (cooking, medical conditions, shopping, etc.). Interestingly, when the patients used the alphabetical strategy, they had the highest accuracy and shortest latencies, yet they preferred the other two strategies and used the alphabetical strategy very little when all three were available. Thus the patients' strategy selection was suboptimal. It is unknown whether they were impaired relative to uninjured controls as the strategy selections made by normal subjects were not presented.
In a second study, a group of TBI patients were found to have deficits in decision making strategies compared with matched controls in a computer-simulated betting exercise (Salmond et al., 2005). The subjects were asked to bet on 1 of 2 options, the odds varied across sessions, and then once they had decided what to bet on, they were given a sequence of choices for how much to bet. The patients took longer to deliberate when deciding what to bet on, were less accurate at determining the option with the best odds of winning, and were more impulsive when deciding how much to bet relative to uninjured controls.
In a third report, patients with TBI and matched controls were asked to memorize a list of noun pairs, and then select a subset of the noun pairs for restudy (Kennedy et al., 2003). The TBI patients appeared worse than controls at selecting which items to restudy. This was interpreted as possibly being due to an impaired self-assessment strategy.
Overall, we conclude that systematic evaluation of behavioral strategies and quantitative analysis of their contributions to overall cognitive performance may be a useful approach to the study of recovery from injury in both experimental animals and human patients.
Acknowledgments
We thank Rich Hartman and David Wozniak for advice on Morris water maze testing, Scott Fujimoto, Valeria Conte, and Tracy McIntosh for performing experimental traumatic brain injury, Steven Paul and Kelly Bales for providing PDAPP mice, and Rich Hartman, Anne Fagan, and the Holtzman lab for helpful discussions. Supported by NIH NS049237, AG13956, AG 20222, a Career Award in the Biomedical Sciences from the Burroughs Wellcome Fund, and Eli Lilly and Co.
References
- Arendash GW, Gordon MN, Diamond DM, Austin LA, Hatcher JM, Jantzen P, DiCarlo G, Wilcock D, Morgan D. Behavioral assessment of Alzheimer's transgenic mice following long-term Abeta vaccination: task specificity and correlations between Abeta deposition and spatial memory. DNA Cell Biol. 2001;20:737–744. doi: 10.1089/10445490152717604. [DOI] [PubMed] [Google Scholar]
- Burke R, Beukelman DR, Hux K. Accuracy, efficiency and preferences of survivors of traumatic brain injury when using three organization strategies to retrieve words. Brain Inj. 2004;18:497–507. doi: 10.1080/02699050310001645784. [DOI] [PubMed] [Google Scholar]
- Chen KS, Masliah E, Grajeda H, Guido T, Huang J, Khan K, Motter R, Soriano F, Games D. Neurodegenerative Alzheimer-like pathology in PDAPP 717V→F transgenic mice. Prog Brain Res. 1998;117:327–334. [PubMed] [Google Scholar]
- Chen G, Chen KS, Knox J, Inglis J, Bernard A, Martin SJ, Justice A, McConlogue L, Games D, Freedman SB, Morris RG. A learning deficit related to age and beta-amyloid plaques in a mouse model of Alzheimer's disease. Nature. 2000;408:975–979. doi: 10.1038/35050103. [DOI] [PubMed] [Google Scholar]
- Colombo PJ, Brightwell JJ, Countryman RA. Cognitive strategy-specific increases in phosphorylated cAMP response element-binding protein and c-Fos in the hippocampus and dorsal striatum. J Neurosci. 2003;23:3547–3554. doi: 10.1523/JNEUROSCI.23-08-03547.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawley JN. Whats Wrong with My Mouse? Chapter 6 Learning and Memory. Wiley-Liss; New York: 2000. [Google Scholar]
- Dixon CE, Clifton GL, Lighthall JW, Yaghmai AA, Hayes RL. A controlled cortical impact model of traumatic brain injury in the rat. J Neurosci Methods. 1991;39:253–262. doi: 10.1016/0165-0270(91)90104-8. [DOI] [PubMed] [Google Scholar]
- Dodart JC, Meziane H, Mathis C, Bales KR, Paul SM, Ungerer A. Behavioral disturbances in transgenic mice overexpressing the V717F beta-amyloid precursor protein. Behav Neurosci. 1999;113:982–990. doi: 10.1037//0735-7044.113.5.982. [DOI] [PubMed] [Google Scholar]
- Dodart JC, Mathis C, Bales KR, Paul SM, Ungerer A. Behavioral deficits in APP(V717F) transgenic mice deficient for the apolipoprotein E gene. NeuroReport. 2000;11:603–607. doi: 10.1097/00001756-200002280-00034. [DOI] [PubMed] [Google Scholar]
- Dodart JC, Bales KR, Gannon KS, Greene SJ, DeMattos RB, Mathis C, DeLong CA, Wu S, Wu X, Holtzman DM, Paul SM. Immunization reverses memory deficits without reducing brain Abeta burden in Alzheimer's disease model. Nat Neurosci. 2002;5:452–457. doi: 10.1038/nn842. [DOI] [PubMed] [Google Scholar]
- Fujimoto ST, Longhi L, Saatman KE, Conte V, Stocchetti N, McIntosh TK. Motor and cognitive function evaluation following experimental traumatic brain injury. Neurosci Biobehav Rev. 2004;28:365–378. doi: 10.1016/j.neubiorev.2004.06.002. [DOI] [PubMed] [Google Scholar]
- Games D, Adams D, Alessandrini R, Barbour R, Berthelette P, Blackwell C, Carr T, Clemens J, Donaldson T, Gillespie F, et al. Alzheimer-type neuropathology in transgenic mice overexpressing V717F beta-amyloid precursor protein. Nature. 1995;373:523–527. doi: 10.1038/373523a0. [DOI] [PubMed] [Google Scholar]
- Graziano A, Petrosini L, Bartoletti A. Automatic recognition of explorative strategies in the Morris water maze. J Neurosci Methods. 2003;130:33–44. doi: 10.1016/s0165-0270(03)00187-0. [DOI] [PubMed] [Google Scholar]
- Grootendorst J, de Kloet ER, Dalm S, Oitzl MS. Reversal of cognitive deficit of apolipoprotein E knockout mice after repeated exposure to a common environmental experience. Neuroscience. 2001;108:237–247. doi: 10.1016/s0306-4522(01)00412-2. [DOI] [PubMed] [Google Scholar]
- Hartman RE, Laurer H, Longhi L, Bales KR, Paul SM, McIntosh TK, Holtzman DM. Apolipoprotein E4 influences amyloid deposition but not cell loss after traumatic brain injury in a mouse model of Alzheimer's disease. J Neurosci. 2002;22:10083–10087. doi: 10.1523/JNEUROSCI.22-23-10083.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebert LE, Scherr PA, Bienias JL, Bennett DA, Evans DA. Alzheimer disease in the US population: prevalence estimates using the 2000 census. Arch Neurol. 2003;60:1119–1122. doi: 10.1001/archneur.60.8.1119. [DOI] [PubMed] [Google Scholar]
- Huitron-Resendiz S, Sanchez-Alavez M, Gallegos R, Berg G, Crawford E, Giacchino JL, Games D, Henriksen SJ, Criado JR. Age-independent and age-related deficits in visuospatial learning, sleep-wake states, thermoregulation and motor activity in PDAPP mice. Brain Res. 2002;928:126–137. doi: 10.1016/s0006-8993(01)03373-x. [DOI] [PubMed] [Google Scholar]
- Irizarry MC, Soriano F, McNamara M, Page KJ, Schenk D, Games D, Hyman BT. Aβ deposition is associated with neuropil changes, but not with overt neuronal loss in the human amyloid precursor protein V717F (PDAPP) transgenic mouse. J Neurosci. 1997;17:7053–7059. doi: 10.1523/JNEUROSCI.17-18-07053.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janus C. Search strategies used by APP transgenic mice during navigation in the Morris water maze. Learn Mem. 2004;11:337–346. doi: 10.1101/lm.70104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy MR, Carney E, Peters SM. Predictions of recall and study strategy decisions after diffuse brain injury. Brain Inj. 2003;17:1043–1064. doi: 10.1080/0269905031000110436. [DOI] [PubMed] [Google Scholar]
- Kotilinek LA, Bacskai B, Westerman M, Kawarabayashi T, Younkin L, Hyman BT, Younkin S, Ashe KH. Reversible memory loss in a mouse transgenic model of Alzheimer's disease. J Neurosci. 2002;22:6331–6335. doi: 10.1523/JNEUROSCI.22-15-06331.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang UE, Lang F, Richter K, Vallon V, Lipp HP, Schnermann J, Wolfer DP. Emotional instability but intact spatial cognition in adenosine receptor 1 knock out mice. Behav Brain Res. 2003;145:179–188. doi: 10.1016/s0166-4328(03)00108-6. [DOI] [PubMed] [Google Scholar]
- Morris R. Developments of a water-maze procedure for studying spatial learning in the rat. J Neurosci Methods. 1984;11:47–60. doi: 10.1016/0165-0270(84)90007-4. [DOI] [PubMed] [Google Scholar]
- Morris RG. Synaptic plasticity and learning: selective impairment of learning rats and blockade of long-term potentiation in vivo by the N-methyl-D-aspartate receptor antagonist AP5. J Neurosci. 1989;9:3040–3057. doi: 10.1523/JNEUROSCI.09-09-03040.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris RG, Garrud P, Rawlins JN, O'Keefe J. Place navigation impaired in rats with hippocampal lesions. Nature. 1982;297:681–683. doi: 10.1038/297681a0. [DOI] [PubMed] [Google Scholar]
- Mortimer JA, van Duijn CM, Chandra V, Fratiglioni L, Graves AB, Heyman A, Jorm AF, Kokmen E, Kondo K, Rocca WA. Head trauma as a risk factor for Alzheimer's disease: a collaborative re-analysis of case-control studies. EURODEM Risk Factors Research Group. Int J Epidemiol. 1991;20 (Suppl 2):S28–S35. doi: 10.1093/ije/20.supplement_2.s28. [DOI] [PubMed] [Google Scholar]
- Murai H, Pierce JE, Raghupathi R, Smith DH, Saatman KE, Trojanowski JQ, Lee VM, Loring JF, Eckman C, Younkin S, McIntosh TK. Twofold overexpression of human beta-amyloid precursor proteins in transgenic mice does not affect the neuromotor, cognitive, or neurodegenerative sequelae following experimental brain injury. J Comp Neurol. 1998;392:428–438. doi: 10.1002/(sici)1096-9861(19980323)392:4<428::aid-cne2>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- Nakagawa Y, Nakamura M, McIntosh TK, Rodriguez A, Berlin JA, Smith DH, Saatman KE, Raghupathi R, Clemens J, Saido TC, Schmidt ML, Lee VM, Trojanowski JQ. Traumatic brain injury in young, amyloid-beta peptide overexpressing transgenic mice induces marked ipsilateral hippocampal atrophy and diminished Abeta deposition during aging. J Comp Neurol. 1999;411:390–398. [PubMed] [Google Scholar]
- Plassman BL, Havlik RJ, Steffens DC, Helms MJ, Newman TN, Drosdick D, Phillips C, Gau BA, Welsh-Bohmer KA, Burke JR, Guralnik JM, Breitner JC. Documented head injury in early adulthood and risk of Alzheimer's disease and other dementias. Neurology. 2000;55:1158–1166. doi: 10.1212/wnl.55.8.1158. [DOI] [PubMed] [Google Scholar]
- Saber AJ, Cain DP. Combined beta-adrenergic and cholinergic antagonism produces behavioral and cognitive impairments in the water maze, implications for Alzheimer disease and pharmacotherapy with beta-adrenergic antagonists. Neuropsychopharmacology. 2003;28:1247–1256. doi: 10.1038/sj.npp.1300163. [DOI] [PubMed] [Google Scholar]
- Salmond CH, Menon DK, Chatfield DA, Pickard JD, Sahakian BJ. Deficits in decision-making in head injury survivors. J Neurotrauma. 2005;22:613–622. doi: 10.1089/neu.2005.22.613. [DOI] [PubMed] [Google Scholar]
- Smith DH, Soares HD, Pierce JS, Perlman KG, Saatman KE, Meaney DF, Dixon CE, McIntosh TK. A model of parasagittal controlled cortical impact in the mouse: cognitive and histopathologic effects. J Neurotrauma. 1995;12:169–178. doi: 10.1089/neu.1995.12.169. [DOI] [PubMed] [Google Scholar]
- Smith DH, Nakamura M, McIntosh TK, Wang J, Rodriguez A, Chen XH, Raghupathi R, Saatman KE, Clemens J, Schmidt ML, Lee VM, Trojanowski JQ. Brain trauma induces massive hippocampal neuron death linked to a surge in beta-amyloid levels in mice overexpressing mutant amyloid precursor protein. Am J Pathol. 1998;153:1005–1010. doi: 10.1016/s0002-9440(10)65643-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutherland RJ, Whishaw IQ, Regehr JC. Cholinergic receptor blockade impairs spatial localization by use of distal cues in the rat. J Comp Physiol Psychol. 1982;96:563–573. doi: 10.1037/h0077914. [DOI] [PubMed] [Google Scholar]
- Thurman DJ, Alverson C, Dunn KA, Guerrero J, Sniezek JE. Traumatic brain injury in the United States: a public health perspective. J Head Trauma Rehabil. 1999;14:602–615. doi: 10.1097/00001199-199912000-00009. [DOI] [PubMed] [Google Scholar]
- Whishaw IQ. Dissociating performance and learning deficits on spatial navigation tasks in rats subjected to cholinergic muscarinic blockade. Brain Res Bull. 1989;23:347–358. doi: 10.1016/0361-9230(89)90221-9. [DOI] [PubMed] [Google Scholar]
- Whishaw IQ, Petrie BF. Cholinergic blockade in the rat impairs strategy selection but not learning and retention of nonspatial visual discrimination problems in a swimming pool. Behav Neurosci. 1988;102:662–677. doi: 10.1037//0735-7044.102.5.662. [DOI] [PubMed] [Google Scholar]
- Whishaw IQ, Mittleman G, Bunch ST, Dunnett SB. Impairments in the acquisition, retention and selection of spatial navigation strategies after medial caudate–putamen lesions in rats. Behav Brain Res. 1987;24:125–138. doi: 10.1016/0166-4328(87)90250-6. [DOI] [PubMed] [Google Scholar]
- Wolfer DP, Lipp HP. Dissecting the behaviour of transgenic mice: is it the mutation, the genetic background, or the environment? Exp Physiol. 2000;85:627–634. [PubMed] [Google Scholar]
- Yue DT, Backx PH, Imredy JP. Calcium-sensitive inactivation in the gating of single calcium channels. Science. 1990;250:1735–1738. doi: 10.1126/science.2176745. [DOI] [PubMed] [Google Scholar]


