Abstract
Desulfovibrio desulfuricans G20 grows and reduces 20 mM arsenate to arsenite in lactate-sulfate media. Sequence analysis and experimental data show that D. desulfuricans G20 has one copy of arsC and a complete arsRBCC operon in different locations within the genome. Two mutants of strain G20 with defects in arsenate resistance were generated by nitrosoguanidine mutagenesis. The arsRBCC operons were intact in both mutant strains, but each mutant had one point mutation in the single arsC gene. Mutants transformed with either the arsC1 gene or the arsRBCC operon displayed wild-type arsenate resistance, indicating that the two arsC genes were equivalently functional in the sulfate reducer. The arsC1 gene and arsRBCC operon were also cloned into Escherichia coli DH5α independently, with either DNA fragment conferring increased arsenate resistance. The recombinant arsRBCC operon allowed growth at up to 50 mM arsenate in LB broth. Quantitative PCR analysis of mRNA products showed that the single arsC1 was constitutively expressed, whereas the operon was under the control of the arsR repressor protein. We suggest a model for arsenate detoxification in which the product of the single arsC1 is first used to reduce arsenate. The arsenite formed is then available to induce the arsRBCC operon for more rapid arsenate detoxification.
Arsenic is present in natural systems as arsenite (AsO33−) and arsenate (AsO43−) (18, 23), and both are toxic to microorganisms (17, 23). Due to the natural abundance of arsenic in geologic systems, a variety of environmental microorganisms have developed enzyme-catalyzed arsenic transformation systems (1, 16, 21). Among the microorganisms that live in arsenate-containing environments, sulfate-reducing bacteria are known to carry out arsenate reduction and are likely to be important mediators of this process. Desulfosporosinus auripigmenti can respire arsenate with resultant arsenite production (20). Several members of the order Desulfovibrionales have also been shown to reduce arsenate (16). Desulfomicrobium sp. strain Ben-RB will grow with lactate and arsenate, while another arsenate-reducing strain, Desulfovibrio sp. strain Ben-RA, cannot grow with arsenate as an electron acceptor, suggesting that arsenate reduction is simply a mechanism for detoxification by strain Ben-RA (16). It is still unclear whether the ability to reduce arsenate is universally present in members of sulfate-reducing bacteria and whether arsenate reduction is typically used for respiration or detoxification.
The best-studied arsenate resistance system in prokaryotes is the ars operon. The ars operon on the Escherichia coli plasmid R773 (the most thoroughly studied ars operon) contains the genes arsRDABC in that order (3, 4, 8, 29). However, the majority of ars operons contain only arsRBC (33). The protein product of arsR is a trans-acting repressor that binds the operon's promoter and shuts down transcription of the operon. Arsenite is a known inducer of the ars operon, acting by inactivation of ArsR (28). The gene product of arsD is a metallochaperone transferring arsenite to ArsA (14). The arsA gene encodes a catalytic subunit of an oxyanion-translocating ATPase (26, 27). The arsB gene encodes a membrane protein that can function independently as a chemiosmotic arsenite transporter. The ArsA/ArsB complex can also be the primary ATP-driven arsenite transporter (7). The protein product of arsC is the cytoplasmic arsenate reductase, which converts intracellular arsenate to arsenite (2, 10). The arsC genes can be divided mainly into two families. The products of the arsC gene from the E. coli plasmid R773 family use glutaredoxin as a reductant (9), while gene products of pI258 and the Bacillus subtilis family use thioredoxin as a reductant (32). Protein sequences between the two families have less than 20% similarity to each other (33). The effect of the ars operon is cumulative, and multiple copies of the ars operon have been shown to increase resistance to arsenate (3).
The ars system is a detoxification system and is thought not to be involved in respiration (17, 33). However, a number of anaerobic microorganisms have another enzyme system that allows them to respire arsenate to arsenite. This system can be the only arsenate-transforming system or it may be present in addition to the above-mentioned detoxification system (30). The best characterized of these arsenate respiration systems contains the arrAB operon. The arrA gene encodes a molybdenum-containing enzyme within the dimethyl sulfoxide reductase family, and arrB encodes an iron-sulfur protein (30, 31).
In this report, the mechanisms for arsenate reduction in Desulfovibrio desulfuricans G20 were investigated. This strain is a genetically tractable derivative of the wild-type strain G100A, which was originally isolated from an oil well corrosion site (35) and subsequently used as a model for sulfate-reducing bacteria. (NCBI genome accession number, NZ_AABN00000000). Here, we have characterized the arsenate transformation system in strain G20 and propose a regulatory mechanism for arsenate detoxification.
MATERIALS AND METHODS
Bacterial strains, plasmids, and growth media and conditions.
D. desulfuricans G20 and pSC27 (35) were obtained from Judy Wall, University of Missouri, Columbia. The anaerobic lactate-sulfate medium used for growth of D. desulfuricans G20 was prepared as previously described (12) under N2 and contained sodium lactate (62 mM), Na2SO4 (50 mM), MgSO4 (8 mM), NH4Cl (5 mM), HEPES (25 mM), K2HPO4 (2.2 mM), CaCl2.2H2O (0.6 mM), yeast extract (0.1%), and trace amounts of other minerals and vitamins (34). The pH was adjusted with NaOH to 7.2 prior to autoclaving. Agar (1.5%) was added to the solid media. NaHCO3 (8 mM) and 0.025% cysteine HCl (for liquid media) or 0.005% PdCl2 (for solid media) was added after autoclaving. The last two were added as reductants. Solid media were dispensed after the addition of antibiotics. Plates were cooled, dried overnight in a laminar-flow hood, and reduced overnight in an anaerobic glove box (Coy Laboratory Products, Inc., Grass Lake, MI). A lactate-sulfate medium containing between 1 μM and 1 mM arsenite (NaAsO2) or between 1 mM and 50 mM arsenate (Na3AsO4) was used for determination of arsenic resistance. The lactate-arsenate medium was similar to the lactate-sulfate medium except that Na3AsO4 (20 mM for liquid or 25 mM for solid media) was substituted for Na2SO4 and MgCl2 was substituted for MgSO4. Arsenic from filter-sterilized stock solutions was added to the media after autoclaving. Typically, 0.1 ml of a 24-h growing culture was inoculated into 10 ml of sterile medium. Kanamycin was added to all media (1,050 μg/ml for liquid media; 175 μg/ml for solid media) used for screening and maintaining G20 transformants (G20 has displayed increased kanamycin resistance in liquid medium). E. coli strain DH5α was grown in Luria-Bertani (LB) broth with 100 μg/ml kanamycin (if needed). LB broth with arsenate concentrations ranging from 1 to 20 mM was used for determining levels of arsenate resistance in E. coli. All cultures were incubated at 37°C, and cell densities in cultures were determined in a Spectronic-20 spectrophotometer and reported as optical density at 600 nm (OD600).
Arsenite detection and arsenite and arsenate quantification.
Arsenite was detected qualitatively by adjusting the pH of sulfate-grown cultures to 3.0 with HCl, allowing orpiment (As2S3) to form (20, 21). Cultures grown without sulfate were treated with 0.1 ml of a solution of 2% Na2S·9H2O and subsequently adjusted to pH 3.0. Arsenate and arsenite were quantified spectrophotometrically using the method of Johnson and Pilson (11).
Genome sequence and phylogenetic analysis.
The D. desulfuricans G20 genomic sequence, along with other proteobacterial sequences, was obtained from http://genome.jgi-psf.org/mic_home.html; alignment and other bioinformatic analyses were carried out with CLUSTALW 1.82 and NNPP promoter finder 2.2 and through the NCBI website (http://www.ncbi.nlm.nih.gov), the BCM searchlauncher website (http://searchlauncher.bcm.tmc.edu), and VIMSS computational genomics (http://www.microbesonline.org).
Analysis of the arsRBCC operon.
In order to determine the start of the arsRBCC operon, rapid amplification of 5′ cDNA ends (5′ RACE) was performed with the 5′/3′ RACE kit (Roche, Mannheim, Germany). Cells were grown in lactate-sulfate medium to an OD600 of 0.2. Then, the cells were treated with 20 mM arsenate to increase expression of the arsRBCC operon. Total RNA was isolated at an OD600 of 0.4 with the RNeasy minikit (QIAGEN Inc., Valencia, CA) following the kit's manual. RNA (1 μg) was used to synthesize the single-stranded cDNA with ParsGSP1 (Table 1) and transcriptor reverse transcriptase. The single-stranded cDNAs were cleaned with the High Pure PCR purification kit (included in the 5′/3′ kit), and a poly(A) tail was added at the 5′ end, using terminal transferase. Then, two rounds of PCR were performed with the cDNA using Taq polymerase and ParsGSP2 and PdTanchor (Table 1) (first round) and ParsGSP3 and Panchor (Table 1) (second round). PCR products were purified with the High Pure PCR purification kit and sequenced with ParsGSP3 and Panchor (Table 1).
TABLE 1.
Primers used in this study
| Primer | Sequence |
|---|---|
| PlinkRB | 5′-AAGGTCCATGCCTTCCTCC-3′/5′-TCTTTGTCACTATCTCGAAACCAA-3′ |
| PlinkBC | 5′-AGGTCCATGCCTTCCTCC-3′/5′-CCCGTGCTCATAGCCCTA-3′ |
| PlinkCC | 5′-CCGCCAAAGGGTTTAGTC-3′/5′-CGGGAGACAAGAAGAAACTC-3′ |
| ParsGSP1 | 5′-GAAGCTGGATGACCTTCTCG-3′ |
| ParsGSP2 | 5′-GAACGGGATGCCAAGATAGA-3′ |
| ParsGSP3 | 5′-CTTGACGTCCGGGTACAGAT-3′ |
| PdTanchor | 5′-GACCACGCGTATCGATGTCGACTTTTTTTTTTTTV-3′ |
| Panchor | 5′-GACCACGCGTATCGATGTCGAC-3′ |
| ParsC1 | 5′-GGTTCATGTTCGCCTCTGCC-3′/5′-GGCCGTCTCTTTCGCGCTG-3′ |
| ParsRBCC | 5′-CCATTAGCTTGTTGCTACACC-3′/5′-GCCAAGGTGCTAATGAAATGA-3′ |
| ParsC1RTa | 5′-GGTTCATGTTCGCCTCTGCC-3′ |
| ParsRRTa | 5′-CGCAGCACGTTCAAAACTC-3′ |
| ParsBRTa | 5′-GACATGACAGACACCGGCAA-3′ |
| ParsC2RTa | 5′-CACCGAATTTGTCACAACGC-3′ |
| ParsC3RTa | 5′-TCGATGGTCTTTGACTCTTGC-3′ |
| P16SRTa | 5′-CTGCTGGCACGGAGTTAGC-3′ |
| ParsC1RealTb | 5′-GTCAAAGCTGGTTTCCGAACTG-3′/5′-GGCTTTCGGCAGGAAAAAA-3′ |
| ParsRRealTb | 5′-TCACAGAAGCCTTAGCTTTGC-3′/5′-ATCCATTTTCCGCTCCTTCT-3′ |
| ParsBRealTb | 5′-ATCGCCATCGGCTTGATTC-3′/5′-GACAATGCGAGCACCTTGAA-3′ |
| ParsC2RealTb | 5′-ATTCCGCTGGTGTGAAAAAG-3′/5′-TCGATGGTCTTTGACTCTTGC-3′ |
| ParsC3RealTb | 5′-ACTCCTGTCGAAGCCAGATG-3′/5′-GGGTTTAGTCCGTGCTTTTTC-3′ |
| P16SRealTb | 5′-GGGTGAGTAACGCGTGGATT-3′/5′-AGCAGAGGCCCCCTTTACC-3′ |
Primers were generated manually based on the sequence at the end of each gene.
Primers were generated by Primer Express (ABI Prism).
In order to be sure that the arsR arsB and arsC2 arsC3 genes belonged to one operon, three sets of primers (PlinkRB, PlinkBC, and PlinkCC) (Table 1) were designed to amplify the three gaps in these four genes. The RNA samples described above (1 μg) were used to synthesize double-stranded cDNA with Superscript reverse transcriptase II and ParsC3RT (Table 1). PCR was performed using the Plink primers and Taq polymerase. The PCR products were checked by gel electrophoresis.
Real-time PCR for quantification of mRNAs.
Cells were grown in lactate-sulfate medium to an OD600 of 0.2. One set of replicate cultures was treated with 20 mM arsenate to determine its effect on induction of mRNA. After the cells had reached an OD600 of 0.4 (about 4 h), cells were harvested by centrifugation (5,000 × g for 5 min at 4°C). RNA was subsequently isolated with the RNeasy minikit. RNA samples (49 μl) were mixed with 8.5 μl DNase and 8.5 μl buffer (Promega Corporation, Madison, WI). The mixtures were incubated at 37°C for 1 h to eliminate the DNA contamination, and RNA was again purified with the QIAGEN RNeasy minikit. For real-time PCR analysis, each of the five ars genes’ cDNAs was first synthesized with 2 pmol gene-specific primers (Table 1), 2 μg total RNA as a template, and 1 μl deoxynucleoside triphosphates (10 mM each). Superscript reverse transcriptase II was used as described in the manual (Invitrogen Corporation, Carlsbad, CA). Real-time PCR was carried out as previously described (24) with the ABI Prism 7000 system (Applied Biosystems, Foster City, CA). Primer sequences were designed by ABI Prism 7000 SDS Software, and amplification was performed by a standard protocol. Each amplicon was 101 bp. Relative quantification of mRNA expression was calculated using the Pfaffl method (24). 16S rRNA was used as a reference gene. DNA contamination of RNA samples was tested by running PCRs as described above but omitting the reverse transcriptase.
MNNG mutagenesis.
D. desulfuricans G20 cells were mutated, and arsenate-sensitive mutants were identified. G20 cells were first grown in lactate-sulfate medium to an OD600 of 0.4. A 1-ml aliquot of cells was treated with 50 μl N-methyl-N′-nitro-nitrosoguanidine (MNNG) solution (2.5 μg/ml) (22) for 4 hours. Surviving cells were recovered on lactate-sulfate plates. The killing rate was 99.7%. Mutants (1,920 colonies) were transferred into parallel 96-well microtiter plates (with and without 20 mM arsenate), and growth was determined after 2 days (13). The ability to reduce arsenate was determined by the formation of a yellow precipitate (orpiment) (19, 20). Potential mutants were subsequently transferred to parallel serum tubes with and without 20 mM arsenate to confirm the loss of arsenate resistance.
General molecular methods.
Plasmids were isolated with the Qiaprep Mini Prep kit (QIAGEN Inc., Valencia, CA). Chromosomal DNA was isolated with the Easy DNA kit (Invitrogen Corp., Carlsbad, CA). PCR was performed by using the Taq DNA polymerase system (Invitrogen Corp.) with Pfu polymerase (Takara Bio Inc., Otsu, Shiga, Japan) to obtain blunt-ended PCR products. T4 DNA ligase (Invitrogen Corporation, Carlsbad, CA) was used to ligate (16 h) the PCR product into pSC27 (100 ng of each). Plasmid constructs were transformed into E. coli DH5α using standard procedures (5). The plasmids were subsequently isolated and transformed into D. desulfuricans G20 as follows. Competent cells of strain G20 were prepared by growing the culture to early stationary phase (OD600, 0.8) and centrifuging the cells under N2 in sealed bottles at 6,000 × g for 10 min at 4°C. The cell pellet was resuspended in 50 ml of an ice-cold solution containing sucrose (400 mM) and magnesium chloride (1 mM) previously sparged with N2 to remove oxygen. This process was repeated twice, and the cells were stored on ice. Cells (85 to 95 μl) were then mixed with 0.5 to 2.5 μg of plasmid DNA to a total volume of 100 μl and treated in an ECM 399 electroporator (BTX Harvard Apparatus Inc., Holliston, MA) at 2,500 V in an anaerobic glove box. Cells were recovered in lactate-sulfate medium for 4 h and then plated out on solid medium with 175 μg/ml kanamycin to select for transformants.
Cloning and sequencing of arsenic resistance genes.
The arsC1 (1.8-kbp) and the arsRBCC operon (2.7-kbp) regions of the chromosomes of these two mutants and strain G20 were amplified with the sequence-specific primers ParsC and ParsRBCC (Table 1). The arsenic resistance genes were cloned into pSC27 as follows. pSC27 was digested with SmaI following the manufacturer's protocol (New England Biolabs, Ipswich, MA), and the 1.8-kbp and 2.7-kbp blunt-ended PCR products were each blunt-end ligated into pSC27. Reconstructed plasmids were chemically transformed (5, 6) into E. coli DH5α by selecting for kanamycin resistance. The plasmids containing PCR products of D. desulfuricans G20's arsenic resistance genes (arsC1 and arsRBCC) were named pXL10c and pXL11op, respectively (Table 1). Genes were sequenced by cloning the same PCR products directly into the pCR4-TOPO vector (TOPO TA cloning kit; Invitrogen Corp., Carlsbad, CA). DNA sequencing was carried out by the dideoxynucleotide chain termination method at the Oklahoma Medical Research Foundation (Oklahoma City, OK).
RESULTS AND DISCUSSION
D. desulfuricans G20 growth and arsenate reduction.
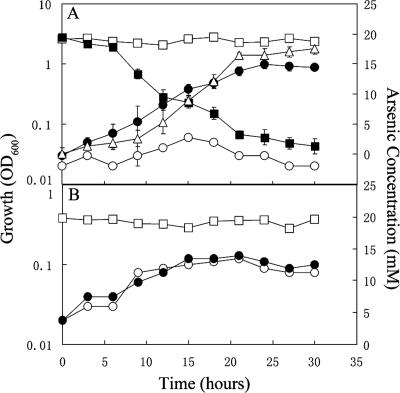
Strain G20 cultures grew and reduced arsenate in liquid media with lactate as an electron donor and sulfate as an electron acceptor at arsenate concentrations up to 20 mM (Fig. 1A). Cultures whose growth was inhibited with erythromycin (Desulfovibrio strains are sensitive to erythromycin; the MIC of strain G20 is 25 μg/ml [unpublished data]) did not detectably reduce any arsenate. G20 cultures typically reach an optical density of 1.1 with or without 20 mM arsenate in lactate-sulfate medium. Although we did not measure lactate consumption or sulfate reduction, it is likely that the 62 mM lactate in the media provided adequate reducing equivalents to reduce all of the arsenate and as much sulfate as was reduced in the control. In the absence of arsenate, the lag phase in lactate-sulfate media was 5.5 h (data not shown) compared to 10.5 h with arsenate (Fig. 1A). Doubling times under both conditions were 3.8 h. This result suggests that arsenate reduction is not an energy-yielding process. The arsenate concentration was reduced to less than 0.1 mM after 24 h (Fig. 1A). Orpiment did not form naturally in these cultures, as either lower sulfide concentrations or lower pH is required for its formation (19). We have also observed that two related species, Desulfovibrio vulgaris Hildenborough and Desulfovibrio sp. strain ASR (a marine strain obtained from Brad Tebo), similarly reduce arsenate (data not shown). Based on our work and previously published studies describing the ability of Desulfovibrio sp. strain Ben-RA and Desulfomicrobium sp. strain Ben-RB (16) to grow with and reduce arsenate, it appears that this ability is likely broadly or universally present within the Desulfovibrionales cluster.
FIG. 1.
Growth and arsenate reduction by D. desulfuricans strain G20. (A) Growth and arsenate reduction in lactate-sulfate medium with 20 mM initial arsenate. Growth (•); arsenate concentration (▪); arsenite concentration (▵). Erythromycin (50 ng/ml) inhibited growth (○) and arsenate reduction (□). The error bars indicate standard deviations. (B) Growth and arsenate reduction in the absence of sulfate. Growth with lactate-arsenate (•); arsenate concentration (□); growth in lactate only medium as a control (○).
The ability of strain G20 to grow and reduce arsenate when present as the sole electron acceptor was also tested. Controls were grown without arsenate. Neither growth nor arsenate reduction was observed (Fig. 1B) under these conditions, suggesting that D. desulfuricans G20 reduces arsenate as a detoxification mechanism rather than as a means of respiration.
When Desulfovibrio sp. strain Ben-RA was grown in lactate-sulfate medium with 9.2 mM arsenate, the arsenate concentration was reduced to about 6 mM after 60 h of incubation, and under the same conditions, Desulfomicrobium sp. strain Ben-RB reduced 8.2 mM arsenate to about 2 mM. The two strains can tolerate arsenate concentrations similar to those tolerated by G20 but exhibit a lower arsenate reduction rate (16). Desulfomicrobium sp. strain Ben-RB also grows with and reduces 3.8 mM arsenate as an electron acceptor in lactate-arsenate medium, a process which neither strain Ben-RA nor strain G20 is able to carry out. This suggests that even though these three strains can reduce arsenate, they may use different mechanisms.
Genome sequence and phylogenetic analysis.
In order to further explore the mechanism of arsenate transformation in D. desulfuricans G20, we searched the genome for both nucleotide and amino acid sequences of arsR, -D, -A, -B, and -C, as well as arrA and arrB from the NCBI nr database. In the D. desulfuricans G20 genome, three open reading frames (ORFs) were identified that displayed similarity to arsCs from pI258 and the B. subtilis family (17, 32): they are designated here arsC1 (gi:23473897; annotated in NCBI), arsC2 (gi:53691679; NCBI), and arsC3 (gi:53691680; NCBI) (Fig. 2). There are five other predicted similar arsC genes within currently available genomic sequences of δ-proteobacteria (see Fig. S1 in the supplemental material) (http://microbesonline.gov). The arsC1 and arsC2 genes are most closely related to each other and similarly related to the arsC genes from Desulfotalea psychrophila LSV54 and Desulfuromonas acetoxidans DSM 684. The arsC3 gene, on the other hand, has only 57% protein similarity to the other two G20 arsC genes and is more closely related to the Wolinella succinogenes arsC (gi:34556458; NCBI) (68% protein similarity) (see Fig. S1 in the supplemental material). Seven δproteobacterial strains have genomic sequences available. Six strains have predicted arsC genes within the pI258/B. subtilis family, while Bdellovibrio bacteriovorus HD100 has a predicted arsC within the R773 family (gi:42521650) (http://microbesonline.gov). The presence of three copies of arsC in this configuration is quite unusual. The only other known multilocus arsenate resistance system is present in Pseudomonas aeruginosa (3, 33). The P. aeruginosa genome contains an arsC gene (accession number, gi:15597475) by itself, while it also has an arsRBC operon (3). These two systems are far apart on the chromosome, as in G20, and both are thought to be functional (33). It is also known that multiple ars resistance genes increase arsenate resistance, based on work with E. coli (3). As arsC is responsible for reduction of cytoplasmic arsenate to arsenite, the presence of three copies of arsC likely provides increased levels of arsenate reduction. This search also revealed one ORF in the G20 genome (gi:23473907; NCBI) whose protein sequence is 86% similar to that of ArsB (gi:116584655) of Bacillus cereus and one ORF whose protein sequence is 68% similar to the putative arsenic efflux pump regulator protein (gi:27464265) of Enterobacter cloacae. Four other predicted arsB genes were detected in genomic sequences available for δproteobacteria: (gi:68001681 in Geobacter metallireducens GS-15, gi:39998045 in Geobacter sulfurreducens PCA, gi:68178276 in Desulfuromonas acetoxidans DSM 684, and gi:50876667 in D. psychrophila LSV54). In addition, three predicted arsR genes were detected in those genomes: (gi:68001683 in G. metallireducens GS-15, gi:39998043 in G. sulfurreducens PCA, and gi:95930854 in D. acetoxidans DSM 684).
FIG. 2.
Diagram showing the genomic locations of arsenate reductase genes of D. desulfuricans. Shown are the locations of the ORFs in the D. desulfuricans genome. The numbers in parentheses are the nucleotide numbers from the NCBI D. desulfuricans genome sequence (NZ_AABN02000000). The base A in boldface is the transcriptional start site of the arsRBCC operon based on 5′ RACE analysis.
The four ORFs, arsC2, arsC3, and those related to arsR and arsB, form a putative arsRBCC operon with an 88% possible promoter upstream of arsR. The arsC1 gene has a 66% possible upstream promoter of its own (Fig. 2). The only other strain of Desulfovibrio in which arsenic resistance has been studied genetically is Desulfovibrio sp. strain Ben-RA (16). In strain Ben-RA, arsenate reduction does not support growth and likely involves a chromosomal gene that hybridizes to arsC of the R773 system (18, 33).
Based on genomic analysis, there are at least three other members of the δproteobacteria with an ars operon in their genomes—G. sulfurreducens PCA (gi:39998043, 39998044, and 39998045), G. metallireducens GS-15 (gi:68001681, 68001682, and 68001683), D. acetoxidans DSM 684 (arsR; gi:68178275 and 68178276)—suggesting a common mechanism of arsenate detoxification among these δproteobacteria. However, the order of the genes in these other ars operons is RCB. D. vulgaris has an arsC homolog and reduces high arsenate levels (data not shown). However, the lack of arsB and arsR indicates that D. vulgaris may utilize a unique mechanism for detoxifying arsenate. There are no strong homologs to arrA and arrB in the G20 genome, providing additional evidence that strain G20 utilizes a detoxification rather than a respiration process to reduce arsenate.
Operon analysis.
In order to prove that the arsRBCC operon detected by sequence analysis is a functional operon, the following tests were carried out. 5′ RACE was performed to determine the transcriptional start of the operon. Sequence analysis has shown that base 331029 (NCBI) is the start of transcription of the arsRBCC operon (Fig. 2). To prove that arsR, arsB, arsC1, and arsC2 are transcribed as a single mRNA, gap amplification PCR was carried out and the PCR products were visualized by gel electrophoresis. Clear PCR products were formed using the PlinkRB, PlinkBC, and PlinkCC primer sets (data not shown), indicating that the entire arsRBCC operon is transcribed as a unit.
Real-time PCR.
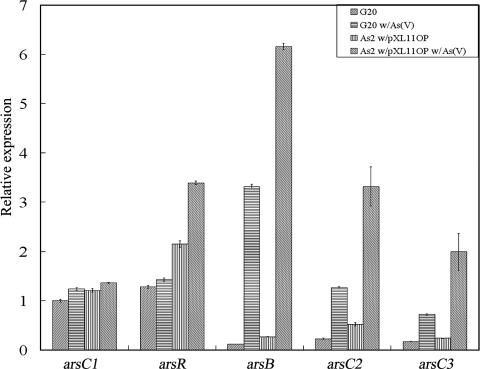
The roles of the two genetic units, arsC1 and the arsRBCC operon, in arsenate resistance remained to be determined. To address this issue, transcriptional analysis was carried out. Expression of arsC1 and arsRBCC was monitored at the transcript level in strain G20 and in the AS2 recombinant (pXL11op) grown with or without arsenate (Fig. 3). In both strains, expression of arsC1 was only marginally increased (24%) upon the addition of arsenate. The regulator arsR was similarly induced by only 18%. On the other hand, expression of arsB was induced about 17-fold and arsC2 and arsC3 were increased about 3-fold (Fig. 3). When transformed with pXL11op, expression patterns were similar but at a slightly higher level (Fig. 3). These results showed that arsB, arsC2, and arsC3 in D. desulfuricans are regulated by arsenic, likely as a direct result of the arsenite produced by reduction of arsenate (28, 29); however, expression of arsC1 appears constitutive. The polar expression effect observed with the arsRBCC operon has been previously reported (15). In the cyanobacterium Synechocystis sp. strain PCC 6803, expression of the arsB gene was increased about 12-fold, while expression of a downstream arsC within the same operon was increased about 2-fold (15). A comparison of expression levels of strain G20 with the pXL11op transformant (Fig. 3) suggests that two or three copies of the plasmid are present in strain G20.
FIG. 3.
Expression levels of arsC1, arsR, arsB, arsC2, and arsC3 during arsenate treatment. G20 w/As(V), G20 treated with arsenate; As2 pXL11op w/As(V), As2 pXL11op treated with arsenate. Expression was determined by real-time PCR and normalized with 16S rRNA. The relative expression of arsC1 in the absence of arsenate treatment was converted to a value of 1 as a reference. The error bars show the standard deviations for triplicate samples.
Characterization of arsenate-sensitive mutants of strain G20.
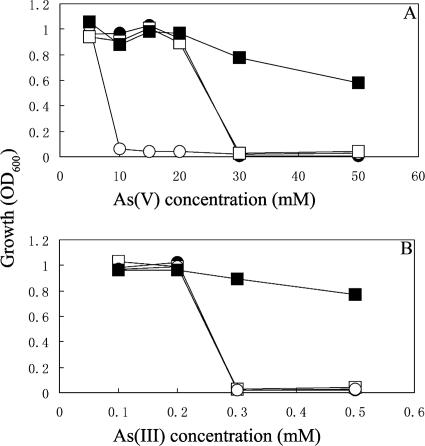
We sequenced the arsRBCC operon and arsC1 of As1 and As2, the two MNNG mutant strains determined to be most sensitive to arsenate. The arsRBCC operons in both mutants are 100% identical to the original strain; there is a single point mutation in each mutant in the arsC1 region, which could have caused dramatic structural changes. In As1, the phenylalanine (TTC) at position 33 has been changed to a serine (TCC), and in As2, the arginine (CGG) at position 64 has been changed to a tryptophan (TGG). These two mutants were subsequently tested for the ability to grow at lower arsenate concentrations. Although neither could tolerate 10 mM arsenate, both could grow at 5 mM arsenate (As1, data not shown; As2, Fig. 4A), likely as a result of residual arsenate reductase activities associated with the intact arsC2 and arsC3. Previous work has shown that the loss of arsC function results in arsenate but not arsenite sensitivity (25). Inactivation of each of the other genes in the arsRBC operon is known to result in different phenotypes (36). Deletion of arsR results in overexpression of arsB and arsC, while the loss of arsB results in sensitivity to both arsenate and arsenite. Both As1 and As2 strains are more sensitive to arsenate than the parental strain but are similarly sensitive to arsenite (Fig. 4B).
FIG. 4.
Max OD600 after 48 h of incubation for recombinant strains of D. desulfuricans with increasing levels of (A) arsenate and (B) arsenite. D. desulfuricans G20 (•); As2 (○); As2 pXL10c (□); As2 pXL11op (▪).
Expression of the cloned arsenate reductase in E. coli DH5α and G20 mutant As2.
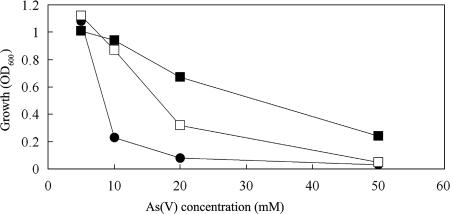
In order to confirm that the identified ars genes were those involved in arsenate reduction in G20, the genes were cloned into E. coli and into the arsenate-sensitive mutant As2. Recombinant strains were subsequently tested to determine whether plasmid-borne genes had conferred arsenate resistance on these strains. E. coli strain DH5α is derived from strain K-12, known to have a chromosomal arsRBC operon (4); however, it would not grow with 20 mM arsenate (Fig. 5). E. coli pXL11op transformants (arsRBCC) were able to grow in LB broth containing 20 mM arsenate to an OD600 of 0.6 in 12 h and tolerated up to 50 mM arsenate with a lower cell density (OD600) (Fig. 5). Orpiment was formed in tubes following growth (after decreasing the pH and addition of sulfide). The pXL10c (arsC1) transformants grew to only a low level with 20 mM arsenate and not at 50 mM (Fig. 5). These transformants lacked additional arsB, the arsenite pump, and therefore were not likely able to remove arsenite as effectively from cells after reduction. In this test, the arsRBCC operon's effect seemed cumulative. A previous study had also shown that introducing an E. coli chromosomal ars operon-containing plasmid into E. coli increased arsenate resistance 2- to 10-fold (3).
FIG. 5.
Maximum growth of E. coli DH5α recombinants in LB broth with arsenate. E. coli DH5α (•); DH5α with pXL10c (□); DH5α with pXL11op (▪).
The two ars gene-containing plasmids were also transformed into the D. desulfuricans mutant As2 (Fig. 4). Although As2 can tolerate only 5 mM arsenate, the complemented mutants (with pXL10c) can tolerate up to 20 mM. It has been shown that MNNG can introduce mutations at multiple sites (22). However, the results of the complementation experiment provide strong evidence that the specific mutation in the arsC1 region of As2 is responsible for loss of arsenate resistance. Surprisingly, As2 complemented with pXL11op can grow at concentrations exceeding 50 mM arsenate (Fig. 4A), suggesting that both copies of arsRBCC are functional in this construct. These results also confirm the fact that both arsC1 and arsRBCC are involved in the response to arsenate toxicity in G20 cells. Mutant As2 pXL10c and strain G20 have similar resistances to arsenite (0.2 mM) (Fig. 4B), confirming that the arsB and arsR genes are intact in As2. The fact that mutant As2(pXL11op) (containing two copies of arsRBCC, one on the chromosome and one on the plasmid) is tolerant of up to 0.8 mM arsenite indicates that additional copies of arsB and arsR can confer extraordinary levels of arsenite resistance.
Freshly inoculated cells were inhibited by an arsenite concentration of 0.3 mM (Fig. 4B), whereas growing cells produced millimolar levels of arsenite that appeared to have little effect on them. It is therefore likely that energized cells are less sensitive to arsenite, as they have the ATP necessary to remove arsenite from cells.
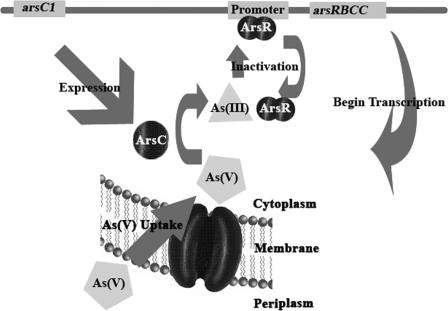
Model for response to arsenate.
Based upon the experimental data, a model is proposed for the observed arsenate reduction response (Fig. 6). In this model, the constitutively expressed arsC1 allows a rapid response to an influx of arsenate into the cell, as the arsenate is reduced by ArsC1. Arsenite formed in the reaction then inactivates ArsR, allowing the arsRBCC operon to be transcribed, with the two copies of arsC allowing high levels of arsenate reduction. It is likely that the multilocus arsenate resistance system in P. aeruginosa is controlled in a manner similar to that of the system described here, although expression studies have not been carried out (3). This model is supported by data showing the need for both As resistance genetic units when cells are treated with 20 mM arsenate. We then predicted that we could bypass the need for arsC1 by pretreating cells with lower levels of arsenate, directly inducing the arsRBCC operon. An experiment was carried out comparing As1 and As2 mutant cells pregrown to an OD600 of 0.1 with or without 5 mM arsenate and then challenged with 20 mM arsenate. In both mutants, cells pretreated with arsenate were able to continue to grow with 20 mM arsenate, whereas cells in which the arsRBCC operon was not induced showed no further growth after the challenge (see Fig. S2 in the supplemental material). The maintenance of a relatively complex arsenate detoxification system such as has been observed here suggests that G20 cells growing in the natural environment must be equipped to deal with rapidly changing and perhaps relatively high levels of arsenate.
FIG. 6.
Proposed model for an arsenate reduction system of D. desulfuricans G20.
Supplementary Material
Acknowledgments
This research was supported by a grant from the Environmental Remediation Science Program (ERSP) of the Office of Biological and Environmental Research of the U.S. Department of Energy Office of Science.
Footnotes
Published ahead of print on 2 March 2007.
Supplemental material for this article may be found at http://jb.asm.org/.
REFERENCES
- 1.Ahmann, D., A. L. Roberts, L. R. Krumholz, and F. M. Morel. 1994. Microbe grows by reducing arsenic. Nature 371:750. [DOI] [PubMed] [Google Scholar]
- 2.Bennett, M. S., Z. Guan, M. Laurberg, and X. D. Su. 2001. Bacillus subtilis arsenate reductase is structurally and functionally similar to low molecular weight protein tyrosine phosphatases. Proc. Natl. Acad. Sci. USA 98:13577-13582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cai, J., K. Salmon, and M. S. DuBow. 1998. A chromosomal ars operon homologue of Pseudomonas aeruginosa confers increased resistance to arsenic and antimony in Escherichia coli. Microbiology 144:2705-2713. [DOI] [PubMed] [Google Scholar]
- 4.Carlin, A., W. Shi, S. Dey, and B. P. Rosen. 1995. The ars operon of Escherichia coli confers arsenical and antimonial resistance. J. Bacteriol. 177:981-986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cohen, S. N., A. C. Chang, and L. Hsu. 1972. Nonchromosomal antibiotic resistance in bacteria: genetic transformation of Escherichia coli by R-factor DNA. Proc. Natl. Acad. Sci. USA 69:2110-2114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Davis, R. W., D. Botstein, and J. R. Roth. 1980. A manual for genetic engineering: advanced bacterial genetics. Cold Spring Harbor Laboratory, Cold Spring Harbor, NY.
- 7.Dey, S., and B. P. Rosen. 1995. Dual mode of energy coupling by the oxyanion-translocating ArsB protein. J. Bacteriol. 177:385-389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Diorio, C., J. Cai, J. Marmor, R. Shinder, and M. S. DuBow. 1995. An Escherichia coli chromosomal ars operon homolog is functional in arsenic detoxification and is conserved in gram-negative bacteria. J. Bacteriol. 177:2050-2056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gladysheva, T. B., K. L. Oden, and B. P. Rosen. 1994. Properties of the arsenate reductase of plasmid R773. Biochemistry 33:7288-7293. [DOI] [PubMed] [Google Scholar]
- 10.Ji, G., and S. Silver. 1992. Reduction of arsenate to arsenite by the ArsC protein of the arsenic resistance operon of Staphylococcus aureus plasmid pI258. Proc. Natl. Acad. Sci. USA 89:9474-9478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Johnson, D. L., and M. E. Pilson. 1975. The oxidation of arsenite in seawater. Environ. Lett. 8:157-171. [DOI] [PubMed] [Google Scholar]
- 12.Krumholz, L. R., and M. P. Bryant. 1986. Eubacterium oxidoreducens sp. nov. requiring H2 or formate to degrade gallate, pyrogallol, phloroglucinol, and quencetin. Arch. Microbiol. 144:8-14. [Google Scholar]
- 13.Kuai, L., A. A. Nair, and M. F. Polz. 2001. Rapid and simple method for the most-probable-number estimation of arsenic-reducing bacteria. Appl. Environ. Microbiol. 67:3168-3173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lin, Y. F., A. R. Walmsley, and B. P. Rosen. 2006. An arsenic metallochaperone for an arsenic detoxification pump. Proc. Natl. Acad. Sci. USA 103:15617-15622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lopez-Maury, L., F. J. Florencio, and J. C. Reyes. 2003. Arsenic sensing and resistance system in the Cyanobacterium synechocystis sp. strain PCC 6803. J. Bacteriol. 185:5363-5371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Macy, J. M., J. M. Santini, B. V. Pauling, A. H. O'Neill, and L. I. Sly. 2000. Two new arsenate/sulfate-reducing bacteria: mechanisms of arsenate reduction. Arch. Microbiol. 173:49-57. [DOI] [PubMed] [Google Scholar]
- 17.Mukhopadhyay, R., and B. P. Rosen. 2002. Arsenate reductases in prokaryotes and eukaryotes. Environ. Health Perspect. 110(Suppl. 5):745-748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mukhopadhyay, R., B. P. Rosen, T. Phung Le, and S. Silver. 2002. Microbial arsenic: from geocycles to genes and enzymes. FEMS Microbiol. Rev. 26:311-325. [DOI] [PubMed] [Google Scholar]
- 19.Newman, D. K., D. Ahmann, and F. M. Morel. 1998. A brief review of microbial arsenate respiration. Geomicrobiology 15:255-268. [Google Scholar]
- 20.Newman, D. K., T. J. Beveridge, and F. M. Morel. 1997. Precipitation of arsenic trisulfide by Desulfotomaculum auripigmentum. Appl. Environ. Microbiol. 63:2022-2028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Newman, D. K., E. K. Kennedy, J. D. Coates, D. Ahmann, D. J. Ellis, D. R. Lovley, and F. M. Morel. 1997. Dissimilatory arsenate and sulfate reduction in Desulfotomaculum auripigmentum sp. nov. Arch. Microbiol. 168:380-388. [DOI] [PubMed] [Google Scholar]
- 22.Oeschger, M. P., and M. K. Berlyn. 1979. A simple procedure for localized mutagenesis using nitrosoguanidine. Mol. Gen. Genet. 134:77-83. [DOI] [PubMed] [Google Scholar]
- 23.Oremland, R. S., and J. F. Stolz. 2003. The ecology of arsenic. Science 300:939-944. [DOI] [PubMed] [Google Scholar]
- 24.Pfaffl, M. W. 2001. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 29:e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rosen, B. P. 2002. Biochemistry of arsenic detoxification. FEBS Lett. 529:86-92. [DOI] [PubMed] [Google Scholar]
- 26.Rosen, B. P. 1999. Families of arsenic transporters. Trends Microbiol. 7:207-212. [DOI] [PubMed] [Google Scholar]
- 27.Rosen, B. P. 2002. Transport and detoxification systems for transition metals, heavy metals and metalloids in eukaryotic and prokaryotic microbes. Comp. Biochem. Physiol. A 133:689-693. [DOI] [PubMed] [Google Scholar]
- 28.Rosenstein, R., K. Nikoleit, and F. Gotz. 1994. Binding of ArsR, the repressor of the Staphylococcus xylosus (pSX267) arsenic resistance operon to a sequence with dyad symmetry within the ars promoter. Mol. Gen. Genet. 242:566-572. [DOI] [PubMed] [Google Scholar]
- 29.Rosenstein, R., A. Peschel, B. Wieland, and F. Gotz. 1992. Expression and regulation of the antimonite, arsenite, and arsenate resistance operon of Staphylococcus xylosus plasmid pSX267. J. Bacteriol. 174:3676-3683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Saltikov, C. W., A. Cifuentes, K. Venkateswaran, and D. K. Newman. 2003. The ars detoxification system is advantageous but not required for arsenate respiration by the genetically tractable Shewanella species strain ANA-3. Appl. Environ. Microbiol. 69:2800-2809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Saltikov, C. W., and D. K. Newman. 2003. Genetic identification of a respiratory arsenate reductase. Proc. Natl. Acad. Sci. USA 100:10983-10988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sato, T., and Y. Kobayashi. 1998. The ars operon in the skin element of Bacillus subtilis confers resistance to arsenate and arsenite. J. Bacteriol. 180:1655-1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Silver, S. 1996. Bacterial resistances to toxic metal ions—a review. Gene 179:9-19. [DOI] [PubMed] [Google Scholar]
- 34.Steger, J. L., C. Vincent, J. D. Ballard, and L. R. Krumholz. 2002. Desulfovibrio sp. genes involved in the respiration of sulfate during metabolism of hydrogen and lactate. Appl. Environ. Microbiol. 68:1932-1937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wall, J. D., T. Murnan, J. Argyle, R. S. English, and B. J. Rapp-Giles. 1996. Transposon mutagenesis in Desulfovibrio desulfuricans: development of a random mutagenesis tool from Tn7. Appl. Environ. Microbiol. 62:3762-3767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wu, J., and B. P. Rosen. 1993. Metalloregulated expression of the ars operon. J. Biol. Chem. 268:52-58. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.