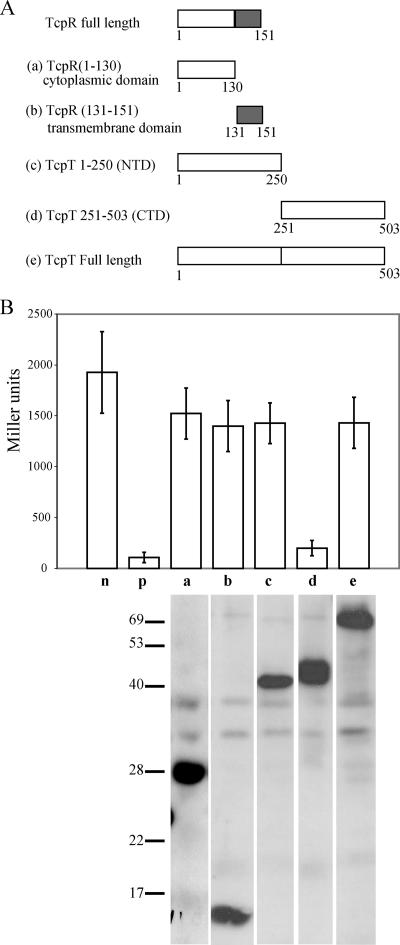
FIG. 7.
The C terminus of TcpT can form multimers, as demonstrated by a LexA-based homologous protein two-hybrid protein system. (A) Representation of various TcpT and TcpR constructs fused to the N terminus of LexA. The letter designated for each of the constructs corresponds to the data shown in panel B. The grey boxes represent the transmembrane domain of TcpR. (B) Analysis of the ability of various TcpT and TcpR constructs to form homodimers. Strains of SU101 were grown to an OD600 of approximately 0.8 in the presence of 1 mM IPTG, and expression of sulA::lacZ was measured by β-galactosidase assays. n, negative control, i.e., empty vector; p, positive control AraC-LexA construct; a, TcpR cytoplasmic domain (amino acids 1 to 130); b, TcpR transmembrane domain (amino acids 131 to 151); c, TcpT N-terminal domain (amino acids 1 to 250); d, TcpT CTD (amino acids 251 to 503); e, TcpT full-length protein. The stability of each of the fusion proteins was detected by anti-LexA immunoblotting, and a composite that includes a representative sample for each strain (the strains were analyzed in triplicate) is shown at the base of the graph for each of the respective strains.