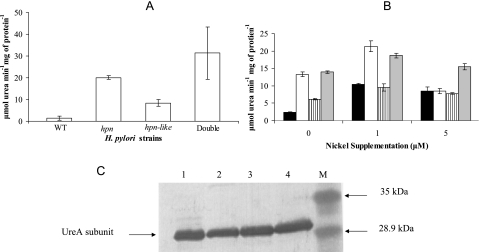
FIG. 2.
Urease activities and protein as a function of the nickel content of the medium. (A) Urease activities of cells grown without nickel supplementation on BA plates. The results shown are means and standard deviations of six replicate samples (these six represent three measurements from each of two independent cultures); the experiment was performed four additional times, with similar results. Among the five experiments, the double-mutant strain mean exceeded the Hpn strain in all cases, by a total range of 5 to 65%. All three mutant strains have significantly greater urease activities than do the wild type (P < 0.01), based on Student t test analysis. (B) Urease activities of cells grown with (1 or 5 μM NiCl2) or without nickel supplementation. Cells were grown on BA plates. The strains are as follows: wild-type H. pylori (black), the hpn strain (white), the hpn-like strain (striped), and the double-mutant strain (gray). The measurements were performed in triplicate (one culture assayed three times); the wild-type urease activities are greater under Ni supplementation conditions than without Ni supplementation (P < 0.01), based on Student t test analysis, and the mutant strain activities exceed the wild-type activity under zero nickel conditions (P < 0.05). The entire experiment was done two other times, with results similar to those shown. (C) Anti-UreA immunoblots performed on gel-resolved peptides of crude cell extracts from cells grown in non-Ni-supplemented medium. Crude extracts were loaded (5 μg protein) with the double-mutant (lane 1), hpn-like (lane 2), hpn (lane 3), or wild type (lane 4) strain. Lane M is the low-range prestained molecular mass marker, with sizes indicated on the right. Densitometric scanning of each lane revealed no significant differences in (UreA) protein amounts between strains.