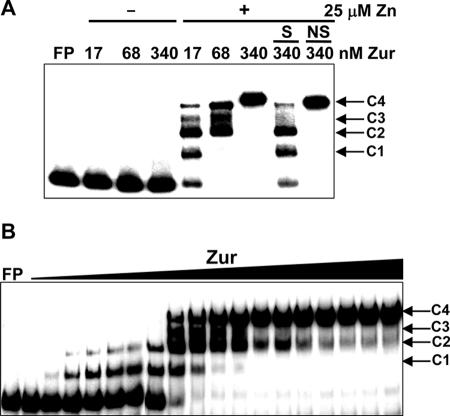
FIG. 3.
Zinc-dependent binding of purified Zur to znuA promoter DNA. (A) Results from gel mobility shift assays in the presence (+) and absence (−) of zinc. Purified Zur protein at various concentrations (from 17 to 340 nM, with increases corresponding to monomeric units) was incubated with 32P-labeled znuA DNA fragments (0.3 nM) in the absence or presence of 25 μM ZnSO4. To confirm the specificity of binding complexes, either a 100-fold molar excess of specific competitors (S; unlabeled znuA DNA) or a 300-fold molar excess of nonspecific competitors (NS; HpaII digest of pGEM-3Zf plasmid) was added to the binding mixture. FP denotes the lane containing the labeled probe DNA only. Four distinct bands with different mobilities were designated C1 to C4. (B) Progressive formation of multiple complex bands (C1 to C4) by increasing amounts of Zur from 0.34 to 340 nM in the presence of 25 μM ZnSO4.