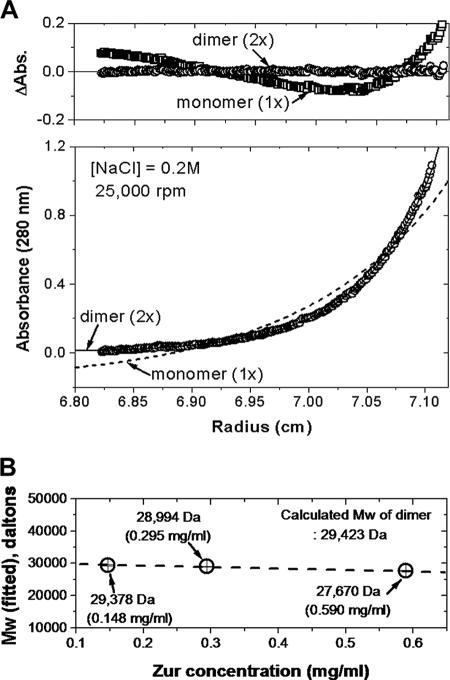
FIG. 4.
Determination of the oligomeric state of Zur by analytical ultracentrifugation. (A) Sedimentation equilibrium of Zur at 25,000 rpm in 20 mM Tris-HCl buffer containing 0.2 M NaCl at 20°C. (Top panel) Distributions of the residuals as a function of the radial positions for monomer and dimer fits. The random distribution of residuals for the dimer fit indicates that Zur is a homogeneous dimer in solution. (Bottom panel) Distributions of absorbance values as a function of the radial position at a protein concentration of 20.1 μM (0.295 mg/ml). The points (circles) represent experimental data obtained at 280 nm, and the fitting lines correspond to a thermodynamically ideal monomer (dotted line) and dimer (solid line). (B) Experimentally determined molecular masses (Mw) of Zur at different protein concentrations: 10.1 μM (0.148 mg/ml), 20.1 μM (0.295 mg/ml), and 40.2 μM (0.590 mg/ml). Standard deviations were smaller than the sizes of the symbols.