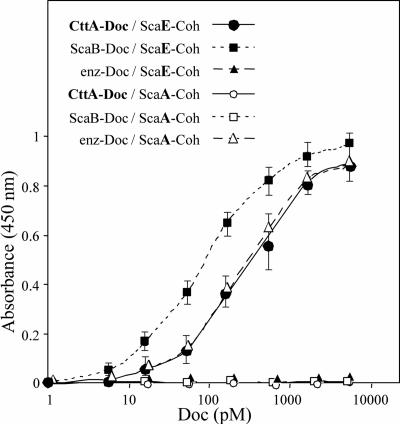
FIG. 5.
Interaction of XDoc dyad from CttA with recombinant cohesin from ScaE. Binding of the CttA XDoc to the ScaE cohesin was achieved using a matching fusion protein system and an affinity-based enzyme-linked immunosorbent assay according to the method of Barak et al. (3). Wells of microtiter plates were coated with the desired CBM-Coh construct (the ScaE cohesin or cohesin 3 from ScaA) at a concentration of 0.3 μM, and the XynDoc fusion proteins, containing XDoc from CttA or ScaB or the dockerin from the R. flavefaciens cellulosomal enzyme Cel44A (enz-Doc), were examined at incremental concentrations. The amount of bound dockerin was determined using a rabbit anti-Xyn antibody followed by a peroxidase-conjugated secondary antibody, and color formation was determined colorimetrically. Error bars indicate the respective standard deviations from the means for the designated points.