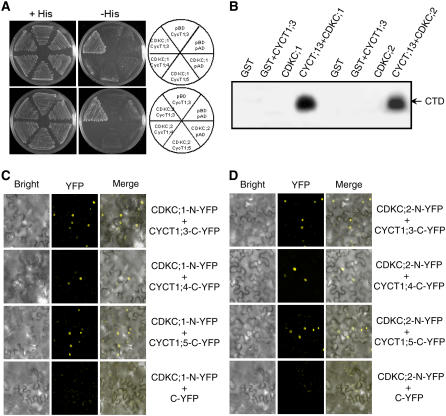
Figure 5.
CDKC;1 and CDKC;2 Interact with CYCT1 Proteins and Phosphorylate the CTD Peptide.
(A) CDKC;1 and CDKC;2 interact with CYCT1;3 but not CYCT1;4 or CYCT1;5 in yeast cells. The Gal4 DNA binding domain–CDKC;1 or –CDKC;2 fusion vector was cotransformed with various activation domain–CYCT1 fusion vectors into yeast cells and grown in nonselective (+His) or selective (−His) medium. The empty DNA binding (pBD) and activation (pAD) vectors were included as negative controls. Growth without added His (−His) demonstrates interaction between protein pairs.
(B) Recombinant glutathione S-transferase (GST), GST-tagged CDKC;1 and CDKC;2, and His-tagged CYCT1;3 proteins were expressed and purified from E. coli. Phosphorylation of the GST-tagged CTD of RNAP II by the indicated proteins was assayed in the presence of [γ-32P]ATP.
(C) and (D) BiFC analysis of CDKC;1 (C) and CDKC;2 (D) interactions in planta with CYCT1 proteins. Yellow fluorescence was observed in the nuclear compartment of N. benthamiana leaf epidermal cells, which results from complementation of the N-terminal part of the YFP fused with CDKC;2 (CDKC;2-N-YFP) with the C-terminal part of the YFP fused with CYCT1;3 (CYCT1;3-C-YFP) or CYCT;5 (CYCT1;5-YFP). No fluorescence was observed when CDKC;1-N-YFP or CDKC;2-N-YFP was coexpressed with unfused C-YFP. Bright-field images (left), YFP epifluorescence images (middle), and overlay images of plasmid autofluorescence and YFP epifluorescence (right) of the same cells are shown.