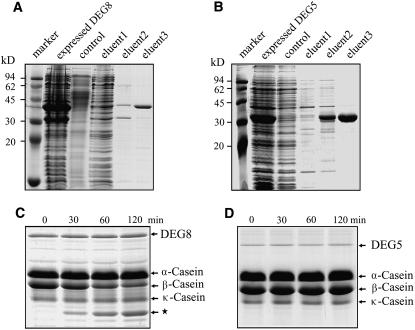
Figure 4.
Purification and β-Casein Degradation Activity of DEG5 and DEG8.
The BL21 cells were harvested after the addition of isopropylthio-β-d-galactoside for 2 h, and the overexpressed DEG8 (A) and DEG5 (B) proteins were purified on a Ni-NTA agarose resin matrix. Samples were resolved by SDS-PAGE and stained with Coomassie blue. The purified proteins from eluent 3 were applied to a Sephadex G-75 column and eluted with 20 mM NaH2PO4, pH 7.8, for renaturation. The purified recombinant DEG8 (0.5 μg) (C) and DEG5 (0.5 μg) (D) proteins were incubated with a mixture of α-, β-, and κ-casein (15 μg) for 30, 60, or 120 min at 37°C. After terminating the reaction, the reaction mixtures were subjected to SDS-PAGE on a 12% (w/v) acrylamide gel. The locations of DEG5, DEG8, and α-, β-, and κ-forms of casein and degradation products (star) in the gel are indicated on the right. Similar results to those presented in (C) and (D) were obtained from three independent experiments. Results from a representative experiment are shown.