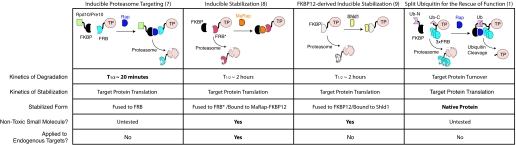
Fig. 1.
A comparison of four methods for the conditional control of proteins using inducible protein degradation. Inducible proteasome targeting uses the FKBP12–rapamycin–FRB system to direct a target protein (TP) for proteasome-mediated degradation upon recruitment of a proteasome subunit (Rpd10 or Pre10). This approach has the fastest kinetics of degradation of the four methods. Inducible stabilization stabilizes target proteins that are otherwise destabilized by fusion to a triple-mutant form of FRB (FRB*) upon binding of FKBP12–rapamycin. This method uses a nontoxic rapamycin analogue called MaRap and has been used for endogenous gene regulation in mice. FKBP12-derived inducible stabilization stabilizes target proteins fused to a mutant form of FKBP12 (FKBP′) with addition of a nontoxic small molecule called Shld1 alone, minimizing disruption of the target protein's activity. SURF restores the native target protein by cleavage of a reconstituted ubiquitin from the target protein upon drug-induced stabilization. FKBP, FKBP12; FRB, FKBP12–rapamycin binding domain; TP, target protein; MaRap, methallyl rapamycin; Ub-N, N-terminal split ubiquitin; Ub-C, C-terminal split ubiquitin.