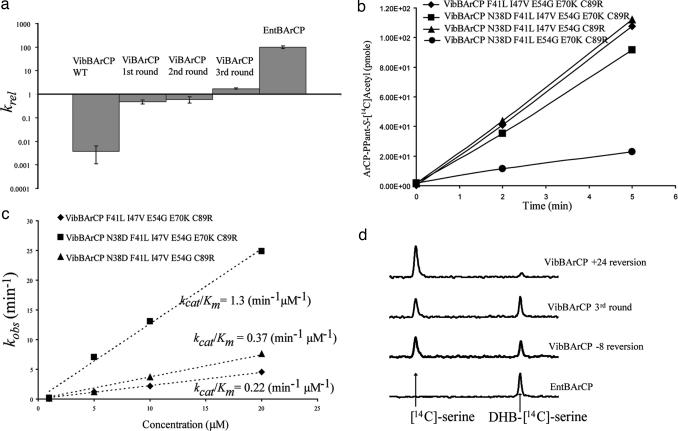
Fig. 4.
In vitro characterization of evolved the VibBArCP mutants. (a) Enterobactin reconstitution assay. Initial rates of enterobactin formation relative to a control experiment using EntBArCP are plotted. After three rounds of evolution, the activity of VibBArCP has been improved by ≈500-fold. (b) Phosphopantetheinylation of VibBArCP (WT and mutants) by Sfp. The ArCPs were incubated with the PPTase Sfp and [1-14C]-acetyl CoA, and the resulting time course for [1-14C]-acetyl-S-ArCP formation was plotted. (c) Acylation of holo-VibBArCPs monitored by incorporation of [14C]salicylate by EntE. The kinetics for different VibBArCP mutants, under kcat/Km conditions, are shown. (d) Single-turnover condensation assay to check interactions between the ArCP domains and EntF. The third-round VibBArCP, and its −8 and +24 reversion mutants were preloaded with DHB, and an EntF tridomain construct (C-A-PCP) lacking the thioesterase domain was loaded with [14C]serine. The HPLC traces show the result of condensation under single-turnover conditions.