Figure 9.
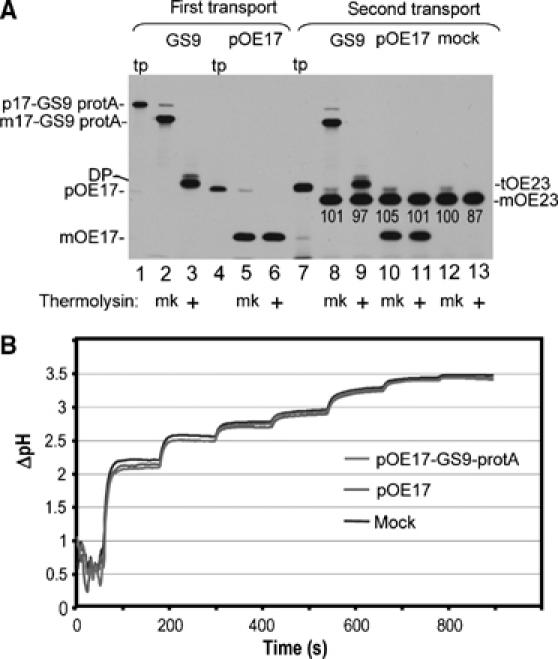
Transport-arrested pOE17-GS9-protA neither interferes with subsequent transport by the cpTat system, nor does it impair the ability to generate and maintain a thylakoidal ΔpH. (A) Chloroplast lysate was incubated with in vitro translated pOE17-GS9-protA (lane 1), pOE17 (lane 4), or mock TnT translation in the light. After 20 min, each assay was supplemented with additional precursor or mock translation extract, and the incubation continued for 15 min. Thylakoids were recovered by centrifugation and washed. Aliquots of thylakoids were analyzed directly (lanes 2 and 5) or thermolysin treated (lanes 3 and 6), or incubated with the cpTat precursor tOE23 (lane 7) in a second transport reaction for 15 min in the light (lanes 8–13). The amounts of pOE17-GS9-protA and pOE17 transported in the first transport incubation were determined by scintillation counting of extracted gel bands and quantitative immunoblotting to be ∼10 000 molecules of mOE17-GS9-protA per chloroplast equivalent and ∼17 000 molecules of mOE17 per chloroplast equivalent. The relative amounts of tOE23 transported in the second incubation (shown below the mOE23 band) were normalized to the amount present in the mock-treated thylakoid assay, which was set at 100. (B) Thylakoids from the first transport incubation (panel A) were examined for the ability to generate a ΔpH (Materials and methods). The light intensities in sequential steps were as follows: 1.5, 3, 5, 6.5, 14, 28, and 50 μE/m2-sec, respectively. Traces represent the average of two independent measurements.
