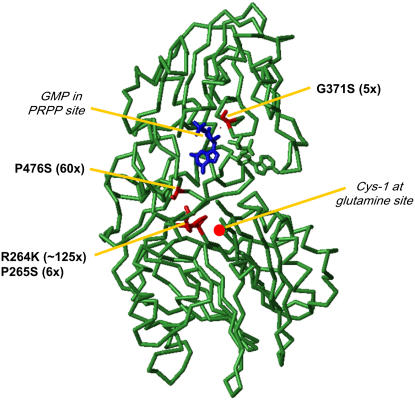
Figure 5.
Position of mutations conferring resistance to DAS734 mapped onto the structure of B. subtilis GPRAT (PDB ID 1AO0) from Chen et al. (1997). The residues in B. subtilis GPRAT that are equivalent to the mutation sites conferring resistance to DAS734 in AtGPRAT2 in the alignment in Supplemental Figure S3 are highlighted in red. The location of the Gln site can be identified by the N-terminal active site Cys residue (red dot). A GMP molecule (in blue) occupies the PRPP catalytic site and an ADP molecule occupies an adjacent allosteric site.