Abstract
The volatile hemiterpene isoprene is emitted from plants and can affect atmospheric chemistry. Although recent studies indicate that isoprene can enhance thermotolerance or quench oxidative stress, the underlying physiological mechanisms are largely unknown. In this work, Arabidopsis (Arabidopsis thaliana), a natural nonemitter of isoprene and the model plant for functional plant analyses, has been constitutively transformed with the isoprene synthase gene (PcISPS) from Grey poplar (Populus x canescens). Overexpression of poplar ISPS in Arabidopsis resulted in isoprene-emitting rosettes that showed transiently enhanced growth rates compared to the wild type under moderate thermal stress. The findings that highest growth rates, higher dimethylallyl diphosphate levels, and enzyme activity were detected in young plants during their vegetative growth phase indicate that enhanced growth of transgenic plants under moderate thermal stress is due to introduced PcISPS. Dynamic gas-exchange studies applying transient cycles of heat stress to the wild type demonstrate clearly that the prime physiological role of isoprene formation in Arabidopsis is not to protect net assimilation from damage against thermal stress, but may instead be to retain the growth potential or coordinated vegetative development of the plant. Hence, this study demonstrates the enormous potential but also the pitfalls of transgenic Arabidopsis (or other nonnatural isoprenoid emitters) in studying isoprene biosynthesis and its biological function(s).
Isoprenoids (also called terpenoids) make up one of the most diverse groups of plant metabolites that not only play several roles between plants and their environment (Pichersky and Gershenzon, 2002), but also have commercial value as, for example, pharmaceuticals and insecticides (Rodriguez-Conception and Boronat, 2002; Aharoni et al., 2006). Isoprene (2-methyl 1,3-butadiene) is the simplest isoprenoid whose function is still relatively unknown even if it is, mainly due to its significant influence on atmospheric chemistry (Thompson, 1992; Biesenthal et al., 1997; Derwent et al., 1998), one of the most studied individual biogenic volatile organic compounds. The global annual isoprene flux from vegetation into the atmosphere is more than half of nonmethane volatile organics emitted globally (Lim et al., 2005). Since Sanadze (1957) first described the capacity of plants to emit isoprene, many different plant species that emit this volatile compound have been described (Kesselmeier and Staudt, 1999). Many woody plants have to date been identified as important isoprene emitters, among which the genera Quercus, Populus, and Salix are of particular importance for Central Europe.
Isoprene is synthesized in plastids through the recently found 2-C-methyl-d-erythritol 4-phosphate (MEP) pathway (Rohmer et al., 1993) that also provides precursors for several other isoprenoid types: mono- and diterpenes, carotenoids, plastoquinones, and the prenyl side chains of chlorophyll. The universal C5 precursors are isopentenyl diphosphate and/or its isomer dimethylallyl diphosphate (DMADP; for review, see Lichtenthaler, 1999). The complete MEP pathway has been elucidated (for overview, see Eisenreich et al., 2001).
Different hypotheses are discussed to explain the role of isoprene emission for the plant itself. Isoprene is considered to function either to prevent leaf metabolic processes from thermal (Sharkey and Singsaas, 1995; Singsaas et al., 1997; for overview, see Sharkey and Yeh, 2001) and oxidative stress (Loreto and Velikova, 2001; Loreto et al., 2001), or to serve as an overflow mechanism for excess of carbon intermediates or photosynthetic energy (Logan et al., 2000; Rosenstiel et al., 2004). In addition to the significant role of isoprene in atmospheric chemistry, it also has commercial value as a precursor for synthetic rubber.
Several studies have been conducted to study isoprene biosynthesis by altering the substrate availability in isoprene emitters with fosmidomycin feeding (Sharkey et al., 2001; Velikova and Loreto, 2005; Velikova et al., 2005) or introducing isoprene emission-like circumstances for non-isoprene emitters with external isoprene fumigation (Sharkey et al., 2001). Either of these methods can mimic the effects of natural isoprene synthesis by the plant, including the effects of isoprene within leaf tissue. However, a significant artifact of fosmidomycin feeding is that it blocks not only isoprene biosynthesis but also the entire MEP pathway, likely resulting in unwanted side effects such as the inhibition of abscisic acid (ABA) biosynthesis (Barta and Loreto, 2006). In addition to the traditional biochemical and physiological approaches, metabolic engineering of isoprenoids is a valuable new tool allowing deeper insight to the biosynthesis and hence the importance of isoprenoid synthesis for plants. Engineering of chloroplastic isoprenoids may be used as a tool to manipulate a large number of traits in plants and provide greater elucidation of the regulation of the MEP pathway. Some approaches modifying the levels of isoprenoid precursors from the MEP pathway have already been completed, leading to, for example, alteration of deoxyxylulose-5-P synthase (DXS), deoxyxylulose-5-P reductase (DXR), and hydroxymethyl butenyl diphosphate reductase (HDR) levels (Estevez et al., 2001; Mahmoud and Croteau, 2001; Botella-Pavia et al., 2004; Carretero-Paulet et al., 2006). Moreover, introducing monoterpene synthases into diverse plants has successfully resulted in changes in emission profiles (Lewinsohn et al., 2001; Lücker et al., 2001; Lavy et al., 2002; Aharoni et al., 2003; Lücker et al., 2004a, 2004b). Isolation of isoprene synthase genes (ISPS) from poplars (Populus spp.; Miller et al., 2001; Sasaki et al., 2005) and kudzu (Pueraria lobata; Sharkey et al., 2005) provided the opportunity to introduce the capacity to synthesize isoprene in normally non-isoprene-producing plants by genetic transformation and thus study the biological function of this simplest isoprenoid with a molecular genetic approach.
The commonly used model plant Arabidopsis (Arabidopsis thaliana) is known not to emit isoprene. However, Arabidopsis, thought to have a relatively simple metabolism, has been lately shown by in silico analysis to have over 30 putative genes belonging to the terpene synthases (TPSs), a multigene family (Aubourg et al., 2002; Chen et al., 2003). Most of these are almost exclusively expressed in flowers (Chen et al., 2003; Tholl et al., 2005; Aharoni et al., 2006), but low terpene emissions from leaves and siliques (Van Poecke et al., 2001; Chen et al., 2003) and even monoterpenes (namely, 1,8-cineole) from roots (Chen et al., 2004; Steeghs et al., 2004) have been detected. The rate of terpene emission from Arabidopsis is comparatively low relative to insect-pollinated species (Chen et al., 2003).
In this work, we created isoprene-emitting Arabidopsis plants expressing constitutively the ISPS gene from Grey poplar (Populus x canescens) cDNA and aimed to verify the proposed “thermotolerance hypothesis” of isoprene. Functional screening and molecular biological as well as biochemical analyses of isolated isoprene-emitting lines were performed. Two new methods, designed especially for fast plant phenotyping and physiological studies of Arabidopsis, were applied: (1) the GROWSCREEN method, recently introduced by Walter et al. (2007), was used to measure subtle alterations in the relative growth rate (RGR) of Arabidopsis; and (2) a newly designed gas-exchange and cuvette system was used to carry out highly dynamic gas-exchange and volatile organic compound (VOC) measurements of Arabidopsis rosettes. Based on biochemical and photosynthetic gas-exchange studies, we discuss the advantages and limitations of isoprene-emitting lines for their use as a model system to address biological functions of isoprene.
RESULTS
Screening of the Transgenic PcISPS-Expressing Arabidopsis Lines
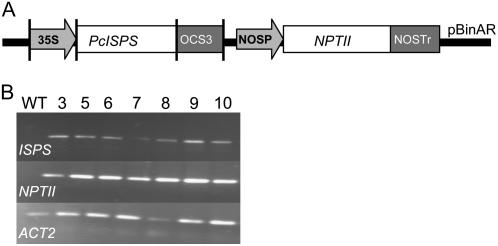
The overall development in size and time to maturity of transgenic plants was comparable to wild-type plants. When seeds (obtained following self-pollination of F1 transformed plants) were plated on Murashige and Skoog with kanamycin, plants appeared to be approximately 75% resistant to antibiotic proofing for single gene insertion. Expression of PcISPS, NPTII, and, for comparison, ACTIN2 (AtACT2) genes from total RNA of F1 generation were measured and testified for successful transformation of Arabidopsis with the poplar ISPS gene (Fig. 1B).
Figure 1.
A, The construct used for generating transgenic isoprene-emitting Arabidopsis plants. B, Accumulation of PcISPS and NPTII in transgenic plants determined by reverse transcription-PCR in seven transgenic lines. Actin (AtACT2) was used for comparison.
Following several isoprene emission measurements from single leaves (F1 [62 individuals screened] and F2 [13 lines screened] generations, age of rosette from 3 to 5 weeks) from plants of transgenic lines (data not shown), five of the lines were selected for further study. Three of the lines (so-called strong isoprene-emitting lines) emitted 3- to 10-fold more isoprene than the two other selected lines (so-called low-emitting lines), whose isoprene emissions were approximately 10-fold stronger than that of the wild type.
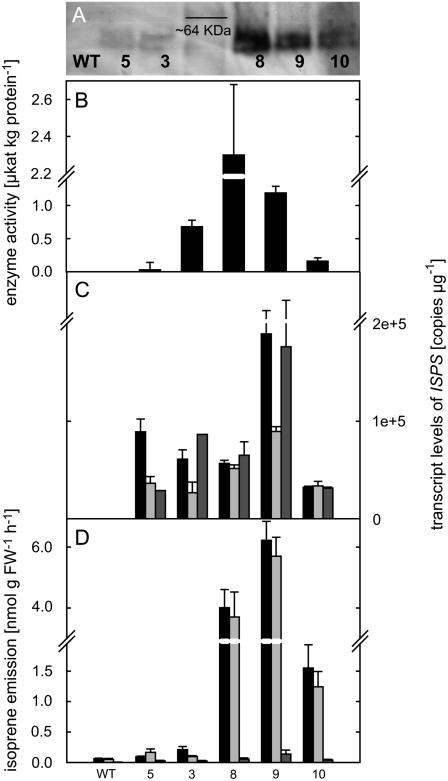
ISPS activity assay was successful only when protein was extracted from fresh plant material of relatively young (2- to 3-week-old) Arabidopsis plants. The strong isoprene-emitting lines (9 and 8; Fig. 2D) had PcISPS activities of 1.2 and 2.4 μkat kg protein−1, respectively, and the other three lines approximately half of that (Fig. 2B).
Figure 2.
Functional analysis of five transgenic PcISPS-expressing Arabidopsis lines (5, 3, 8, 9, and 10; WT = wild type) later used for behavior experiments. A, PcISPS protein revealed in western-blot analysis (20 μg of protein loaded) from each line. B, PcISPS activity (n = 3 ± se) detected from 2-week-old plant material. C, PcISPS transcript level (copies μg−1 RNA) in leaves (black bars), flowers (light-gray bars), and roots (dark-gray bars; n = 2 ± 1); D, isoprene emission rate from single leaves (n = 12 ± se), flowers (including approximately 1 cm stem; n = 12 ± se), and roots (n = 3 ± se). Middle-aged leaves from 5- to 7-week-old plants were used for all the experiments except for enzyme activity that was detected from 2-week-old plants.
ISPS protein concentration correlated with PcISPS activity and isoprene emission levels, being strongest in lines 8 and 9 and lower in the other lines (Fig. 2, A, B, and D).
From wild-type plants, no PcISPS activity or protein signal (Fig. 2, A and B, respectively) could be detected.
PcISPS Expression and Volatile Production from Different Parts of the Plants in Different Stages of Development
Being introduced into the Arabidopsis genome under the regulation of a constitutive promoter, PcISPS was expressed in all organs of Arabidopsis. Four of the five lines tested showed globally similar PcISPS expression level when compared to each other. Only line 9 had approximately 2-fold higher transcript levels than other lines in all plant organs. In general, expression levels in leaves and roots were similar in magnitude, being 2-fold higher than the level in flowers (Fig. 2C). In nontransgenic control plants, PcISPS expression was not detected.
Leaves, roots, and flowers (with approximately 1 cm stem) of transgenic Arabidopsis emit isoprene. Isoprene emission from leaves and flowers was 4 to 6 nmol g fresh weight (FW)−1 h−1 from strong isoprene-emitting lines 8 and 9, and 1.5 nmol g FW−1 h−1 from line 10. Emission from line 3 was 0.2 nmol g FW−1 h−1, being around 4-fold more than that from line 5 or from wild type (Fig. 2D). Even if no PcISPS expression, enzyme activity, or protein signal (Fig. 2, C, B, and A, respectively) could be detected in the wild type, minor isoprene emission from leaves and flowers was observed (Fig. 2D). It is possible that the emissions detected from wild-type plants correspond to degradation products of DMADP or other compounds synthesized by the plants or indeed a by-product of some of the monoterpene synthases in Arabidopsis.
Isoprene emission from roots was low but nonetheless detectable, varying between 0.02 to 0.1 nmol g FW−1 h−1 (Fig. 2D). Highest emissions were again observed in lines 8 and 9. Comparatively, emission rates from line 10 were approximately one-third, whereas rates observed from lines 3 and 5 were approximately only one-quarter of those observed in lines 8 and 9.
Substrate Availability from the MEP Pathway
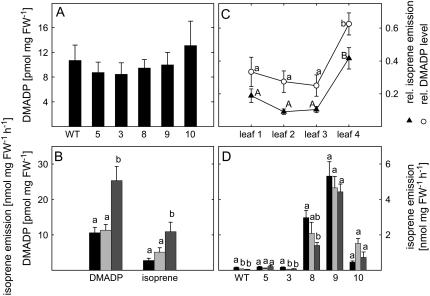
To find a reason for the low level of isoprene emission from transgenic Arabidopsis, the DMADP availability from MEP pathway for isoprene biosynthesis was examined. Despite the need for substrate to produce isoprene, DMADP levels in transgenic plants were not found to be altered and were similar in range with levels in wild type, around 10 pmol mg−1 FW (Fig. 3A). However, when the metabolic flux through the MEP pathway was artificially enhanced with 1-deoxy-d-xylulose (DOX) feeding, DMADP levels were significantly (P < 0.01, independent-samples t test) up-regulated, producing a similar range of enhancement in isoprene emission rates. Feeding with 30 nm DOX resulted in 2-fold higher DMADP level and isoprene emission, whereas feeding with a lower concentration of DOX (3 nm) did not produce any significant changes (Fig. 3B). This result indicates that isoprene emission in transgenic Arabidopsis is substrate limited.
Figure 3.
A, DMADP level in the leaves of whole rosette in transgenic plants (lines 5, 3, 8, 9, and 10) and in the wild type (WT; n = 8 ± se). B, DMADP level and isoprene emission in line 8 in control leaves (black bars), and in leaves fed with 3 nm DOX (light-gray bars) or with 30 nm DOX (dark-gray bars; n = 6 ± se). C, Relative DMADP (○) level in wild-type and transgenic plants (lines 3, 5, 8, 9, and 10; n = 12 ± se) and isoprene emission (▴) in transgenic plants (lines 3, 5, 8, 9, and 10; n = 25 ± se) in leaves having different developmental stages: Leaf 4 represents the youngest, leaf 1 the oldest, and leaves 2 and 3 middle-aged leaves in 5-week-old rosette. D, Isoprene emission from 3-week-old (black bars, n = 12 ± se), 5-week-old (light-gray bars, n = 6 ± se), and 7-week-old (dark-gray bars, n = 12 ± se) Arabidopsis rosettes. Middle-aged leaves from 4- to 6-week-old plants were used if no other mentioned. Letters a and b (DMADP level) or A and B (isoprene emission) indicate statistically significant differences (P < 0.01, Tukey's HSD in B; and P < 0.05, Wilcoxon's paired-samples test in C and D).
The highest DMADP levels as well as the highest isoprene emission rates were found in young, approximately 24-h-old, developing leaves of Arabidopsis rosettes. Both levels were significantly reduced in older leaves compared to young ones (P < 0.01, Wilcoxon's paired-samples test; Fig. 3C). When isoprene emissions from single leaves were measured from 3-, 5-, and 7-week-old Arabidopsis, the highest isoprene emission rate was present in leaves from 3-week-old rosettes in lines 8 and 3 and, surprisingly, also in the wild type (P < 0.05, Wilcoxon's paired-samples test). However, no differences in DMADP level or in isoprene emission were found between 7- and 5-week-old leaves (Fig. 3D). The tendency was similar also in line 9 even if not statistically significant. However, in line 10, highest isoprene emissions occurred in 5-week-old leaves (Fig. 3D).
Measurement of total carotenoid (wild type: 0.224 ± 0.016 mg g FW−1; line 8: 0.227 ± 0.018 mg g FW−1; line 9: 0.23 ± 0.008 mg g FW−1) and chlorophyll (wild type: 1.27 ± 0.11 mg g FW−1; line 8: 1.28 ± 0.12 mg g FW−1; line 9: 1.25 ± 0.04 mg g FW−1) levels showed no difference between transgenic lines and the wild type, indicating that photosynthetic pigment concentrations were not affected by the introduction of the PcISPS gene.
Growth Analysis of Transgenics versus Wild Type under Normal and Thermal Stress Conditions
To understand the influence of isoprene emission for overall plant fitness, experiments to measure shoot growth rate under normal and altered temperature conditions were set up. Two different methods were used to quantify growth rates, using either leaf area or biomass measurements.
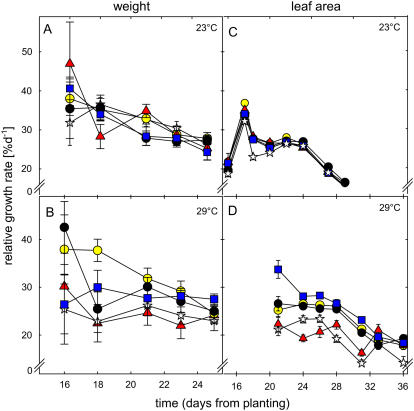
When the leaf area was measured, leaf growth of isoprene-emitting Arabidopsis plants (lines 8, 10, and 3) was significantly faster (ANOVA and Tukey's post hoc analysis, P < 0.05, P < 0.001, and P < 0.01, respectively) compared to the wild type or to the very low isoprene-emitting line 5, between day 20 and day 35 after planting under moderate thermal stress (Fig. 4D). In the beginning of the experiment, lines 3 and 8 grew approximately 30% faster than the wild type, whereas in line 10 this value was around 60% faster. By the end of experiment, the difference had diminished. In the absence of temperature stress (Fig. 4C), differences between lines were smaller, but isoprene-emitting lines still grew faster than the wild type (P < 0.01).
Figure 4.
RGR of transgenic lines 3 (yellow circle), 5 (red triangle), 8 (black circle), and 10 (blue square) and wild type (white star) in 23°C calculated from biomass (A) or from leaf area (C) and at 29°C calculated from biomass (B) or from leaf area (D). The given values are means of (A and B) 12 individuals ± se and means of (C and D) 30 individuals ± se.
When biomass was used to calculate the RGR (Fig. 4, A and B), differences between isoprene-emitting lines and the wild type were of the same order of magnitude as those based on noninvasively measured leaf area data, but variability was higher due to destructive harvests of different populations for data acquisition at different time points. Based on the FW data, only line 3 showed significantly faster growth (ANOVA and Tukey's post hoc analysis, P < 0.001) compared to the wild type or line 5. The lines grown at 23°C did not show differences in RGR compared to the wild type. Differences in RGR decreased throughout the experiment and plants from different lines reached comparable final sizes. Only line 10 reached a final, significantly higher biomass (P < 0.001) under thermal stress compared to the wild type (data not shown). This result is likely due to biomass gained in the first week after germinating, when the line overcame the altered temperature more efficiently. Line 9 was excluded from growth analysis because at that state it showed no homozygous phenotype, even though the line emitted reasonable amounts of isoprene.
Photosynthetic Gas Exchange and Isoprene Emission under Slowly Increasing Temperature Stress and Recovery
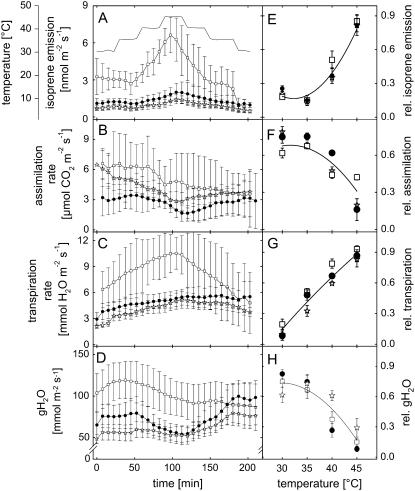
To analyze dynamically photosynthetic gas exchange and VOC emissions from whole plant, a newly developed cuvette system designed especially for Arabidopsis rosettes was used (Fig. 5). The dependency of isoprene emission and photosynthetic parameters on temperature were tested in transgenic and wild-type Arabidopsis with a temperature program increasing leaf temperature in 5°C steps from 30°C until 45°C and back, lasting for 30 min at each temperature plateau. Isoprene emission started to increase in both transgenic lines similarly during the second half of the period at 35°C after which it continuously increased, being twice as high at 45°C compared to the initial values (Fig. 6A). Both transgenic lines showed higher isoprene emission rates than wild-type plants, but the emission was statistically significant only for line 9 (P < 0.01, Tukey's post hoc analysis). Isoprene emission from the transgenic lines also correlated positively with temperature (P < 0.01, Pearson's correlation). Net assimilation decreased with increasing leaf temperature, to approximately half of the initial values in line 8 and the wild type and by two-thirds of initial values in line 9 (Fig. 6B). Similarly, stomatal conductance (gH2O) decreased with increasing leaf temperature up to 40% in line 9 and up to 20% in line 8 of initial values (Fig. 6D). Conversely, leaf transpiration correlated positively with increasing temperature (P < 0.01 but for line 8 P = 0.023, Pearson's correlation), rising to approximately 60% of initial values in all lines tested (Fig. 6C). Line 9 showed significant difference in net assimilation, transpiration, and gH2O compared to the wild type and line 8 (P < 0.05, Tukey's post hoc analysis). The values detected with decreasing leaf temperature were not taken into account because the recovery from the relatively high thermal stress was often not complete (visible damage of plants).
Figure 5.
Pictures of an open (A) and a closed (B) Arabidopsis cuvette. Thermocouples (1) inside of the cuvette measure leaf temperature continuously. In the basement of the cuvette system, the Arabidopsis rosette is fixed and the soil is covered with plastic pieces (2). Ventilators and a cooling element (3) cool down the LED light head (5) and the Peltier element (4). The inlet air port (6) is placed on the opposite of the outlet air port. Shown are pictures of wild-type (C) and transgenic (D) Arabidopsis plants at the age of 2 weeks.
Figure 6.
Isoprene emission (A), net assimilation (B), transpiration (C), and stomatal conductance (gH2O) (D) rates measured online from Arabidopsis rosettes of line 8 (•), line 9 (□), and wild type (open star) when temperature was increased stepwise by 5°C each 30 min from 30°C until 45°C and cooled back to the initial value. The given values are 7.5-min means of three (line 8) to four (line 9 and wild type) individual plants ± se. Relative isoprene emission (E), net assimilation (F), transpiration (G), and stomatal conductance (H) rates correlate with rising temperature. The shown relativized values are 30-min means of three to four individual plants ± se in each temperature.
Relative values (highest value brought to 1 and lowest to 0) were calculated and plotted as 30-min means in each temperature step during the first 120 min. Isoprene emission showed an exponential increase (Fig. 6E) but net assimilation (Fig. 6F) and gH2O (Fig. 6H) exponential decrease in dependence of leaf temperature. Transpiration increased almost linearly with increasing leaf temperature (Fig. 6G). A quadratic curve was fitted on each plot; isoprene emission: y = 4.1622 − 0.2475x + 0.0038x2, R2 = 0.9320; net assimilation: y = (−1.4241) + 0.1329x − 0.0021x2, R2 = 0.9412; transpiration: y = (−1.8947) + 0.0808x − 0.004x2, R2 = 0.9701; gH2O: y = (−1.3563) + 0.1416x − 0.0024x2, R2 = 0.9963.
Impairment of Photosynthetic Gas Exchange under Transient Fast-Changing Thermal Stress and Its Recovery
Temperature experiments to test possible functions of isoprene have previously been performed in a number of different ways. However, when testing the role of isoprene in thermotolerance, it is important to bear in mind that Sharkey and Singsaas (1995) suggested a role of isoprene specifically in the protection of leaves from damage caused by rapid and transient events of high temperature (Singsaas and Sharkey, 1998). To test their theory under conditions that resemble relevant scenarios of heat stress in nature, Sharkey et al. (2001) assessed thermotolerance as recovery of photosynthesis from short-term treatments at 46°C. A comparable approach was chosen in this work to test isoprene function in Arabidopsis rosettes under temperature fluctuations ranging from 30°C to 40°C, which are realistic under natural conditions.
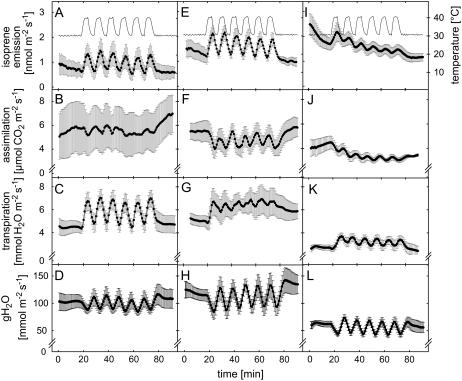
Isoprene emission, net assimilation, and transpiration were measured before, during, and after a transient temperature stress created with rapidly cycling leaf temperature under constant photosynthetic photon flux density (PPFD) of 1,000 μmol photons m−2 s−1 (Fig. 7). Before the start of the six heat cycles, significantly higher isoprene emissions were detected in lines 8 and 9 compared to the wild type. Transpiration and gH2O were higher, but net assimilation showed no differences in line 8 compared to the wild type. In line 9, however, transpiration and gH2O were globally lower than in other lines before the start of heat cycles and through the whole experiment.
Figure 7.
Isoprene emission, net assimilation, transpiration, and stomatal conductance (gH2O) of wild type (A, B, C, and D, respectively), line 8 (E, F, G, and H, respectively), and line 9 (I, J, K, and L, respectively) before, during, and after the temperature stress treatment. After 20 min in 30°C, six heat cycles (temperature switched in 10 min from 30°C to 40°C and back) were performed. The recovery of the plants was measured during 20 min after the heat cycles. The given values are 50-s means of four individual plants ±se.
Heat cycles were started after 20 min of acclimation of gas exchange and isoprene emission. Isoprene emission rates cycled rhythmically with rising and decreasing temperatures in all the plants. Each increase in leaf temperature up to 40°C caused a rapid transient reduction in net assimilation and gH2O accompanied by an increase in transpiration in all the plants. Within 30 min both the wild type and line 8 recovered completely from heat stress cycles without significant reductions in net assimilation and transpiration. Indeed, it seemed that wild-type plants had higher net assimilation rates after the heat stress than before that (P < 0.05, paired-samples t test), whereas in line 8 transpiration increased and gH2O decreased significantly compared to the initial values (P < 0.01, paired-samples t test). However, the initially lower assimilation and isoprene emission levels of line 9 decreased (P < 0.01, paired-samples t test) after the transient thermal stress treatment, indicating that from transient thermal stress the highest isoprene-emitting line survived worse than the wild type or lower isoprene-emitting line 8. Transpiration and gH2O of line 9 recovered completely from heat stress cycles. In general, behavior of line 9 seemed more comparable to the behavior of the wild type than to line 8.
DISCUSSION
Overexpression of the Grey poplar ISPS in Arabidopsis under control of the 35S promoter resulted in functional enzyme and isoprene-emitting Arabidopsis plants. Comparable isoprene-emitting Arabidopsis plants were also produced by Sharkey et al. (2005) introducing a genomic clone of ISPS from kudzu, but no physiological studies with these lines were published. Our investigations showed that isoprene emission is under complex regulation even if the PcISPS gene under control of a constitutive promoter is introduced into a nonnatural isoprene emitter. Only lines 3, 5, and 9 showed patterns of PcISPS expression that explain the isoprene emitted. In lines 8 and 10, the low PcISPS expression could not explain the relatively high ISPS activity and emission. However, even if high discrepancies in isoprene emission and gene expression were detected, the division of these parameters between the plant parts/organs was in principle similar within the line. The discrepancies in gene expression, enzyme activity, and emission levels might be explained by environmental or developmental differences between the plants at the time of harvest.
Since the MEP pathway has only been discovered in the last decade, very little is known about mechanisms of regulation of this important pathway for isoprenoid biosynthesis. Several studies have shown that the gene expression of DXS correlated with the level of carotenoid end products (Estevez et al., 2001; Enfissi et al., 2005) in many plant species. However, regulation is at least to some extent shared with DXR (Carretero-Paulet et al., 2006) and with HDR (Botella-Pavia et al., 2004) genes. DOX feeding bypasses DXS and its regulative role on the MEP pathway, providing substrate for the MEP pathway externally (Wolfertz et al., 2003) and leading in our studies to higher isoprene emission. This result proves that isoprene emission in Arabidopsis depends on the substrate availability from the MEP pathway. Compared to isoprene-emitting species, for example, poplar (Behnke et al., 2007) or oak (Quercus spp.; Brüggemann and Schnitzler, 2002b), leaf DMADP levels in Arabidopsis are lower by a factor of 6 to 10. Taking into account the very high Km (2.45 mm) of poplar ISPS (Schnitzler et al., 2005), it is very likely that isoprene emission in Arabidopsis is substrate limited as indicated by the DOX feeding. The emission of transgenic Arabidopsis is in its maximum approximately 50% of the emission of climate chamber-grown, wild-type poplar (approximately 3 nmol m−2 s−1; Behnke et al., 2007) and ISPS activity approximately in the same order of magnitude with climate chamber-grown poplars (Loivamäki et al., 2007). Compared to field-grown poplar trees, isoprene emission of Arabidopsis reaches in its maximum only 1/30 of their emission level (Behnke et al., 2007).
Aharoni et al. (2003) showed that when Arabidopsis plants were transformed with a limonene synthase gene under regulation of a constitutive promoter, limonene was emitted in a diurnal pattern. Such a feature is likely due to the fact that intermediates are provided diurnally from the MEP pathway (Mayrhofer et al., 2005; Magel et al., 2006) and indicates that isoprenoid production in transgenic plants is not always solely dependent on inserted gene expression and enzyme activity levels, but is regulated in a more complicated matter. With respect to our data, it is likely that the weak isoprene emission in Arabidopsis might be enhanced by co-overexpression of DXS, even though DXR, HDR, and the flow of precursors from the Calvin cycle can still affect DMADP availability.
DXS and DXR gene expression are to some extent similar, having highest levels in developing parts, in light-grown seedlings and in the inflorescence (Carretero-Paulet et al., 2002). Consistent with availability of DXS and DXR, our results revealed the highest DMADP levels in young, developing Arabidopsis leaves. Our data strongly support the idea of developmental regulation of the MEP pathway in Arabidopsis, as the isoprene emission from transgenic plants correlated significantly with DMADP level, being highest in young leaves of the Arabidopsis rosette. In addition, most of the transgenic lines showed higher isoprene emission from leaves of 3-week-old rosettes than from leaves of 7-week-old rosettes. Therefore, substrate availability for isoprenoid biosynthesis at least depends on the developmental stage in Arabidopsis, and an increased production of isoprene might be connected to mechanisms retaining the growth potential of a plant under moderate drought stress situations. Furthermore, ISPS activity was higher in young leaves and could not be detected in old leaves at all, although gene expression of PcISPS was under constitutive control. A similar feature was found by Lücker et al. (2001) in transgenic petunia (Petunia hybrida) plants that constitutively expressed a linalool synthase gene. Enzyme activity could not be detected from old petunia leaves despite the relatively simple detection of activity in young leaves. As a possible explanation, they discussed a technical problem due to different chemical composition in older leaves or simply a lower linalool synthase activity. These observations collectively suggest that it is very difficult to predict isoprene emission from a whole Arabidopsis rosette at a certain developmental stage.
An additional point of interest is the low but undeniable isoprene emission from wild-type Arabidopsis plants. Despite the fact that not all the AtTPS genes have been isolated and characterized, it is unlikely that a gene coding for ISPS could be found. Therefore, we suggest that the detected emissions likely result from the chemical degradation of DMADP or due to side activity of other isoprenoid synthases. An opportunistic question might be whether this low level of isoprene naturally emitted by Arabidopsis could explain why the plants adapt relatively well to the new feature in their genotype and phenotype. Moreover, isoprene is known to be normally emitted from photosynthetically active tissues. Therefore, it would be interesting to know the origin of the small isoprene emission we detected from roots. Transgenic Arabidopsis containing a PcISPS promoter-reporter gene EGFP/GUS construct clearly show promoter activity in Arabidopsis roots (S. Louis and G. Cinege, personal communication), indicating that ISPS gene expression is in principle possible in nonphotosynthetic active tissues.
The introduction of PcISPS into Arabidopsis could have led to a redirection of isoprenoid precursors and, thus, isoprene emission competes with formation of other isoprenoids synthesized downstream of the MEP pathway. So far, many experiments aiming to cause overexpression of isoprenoids or related genes have shown altered phenotypes of transgenic plants being restricted in growth due to depletion of precursors (Fray et al., 1995; Aharoni et al., 2003, 2006). For example, transformation of (S)-linalool and linalool derivatives synthase-encoding genes into Arabidopsis (Aharoni et al., 2003) or into potato (Solanum tuberosum; Aharoni et al., 2006) led to altered phenotypes when higher linalool levels were detected. According to the authors, the change in phenotype might have been observed because of a reduction in precursor availability but could also have been due to a toxicity of the newly synthesized compound. Knowing that isoprene is an energetically expensive product (Magel et al., 2006), one could expect that the need of extra carbon for its biosynthesis also resulted in retarded growth in isoprene-emitting Arabidopsis. Therefore, it is surprising that the opposite was observed, namely, that isoprene-emitting plants grew faster than the wild type under thermal stress. Interestingly, the transgenic Arabidopsis plants grew fastest at the beginning of rosette development when DMADP levels as well as isoprene emission rates were higher and ISPS activity detectable in young plants. Supporting our observations, Carretero-Paulet et al. (2002) showed the highest metabolic flux through the MEP pathway occurred in an early stage of plant development.
Furthermore, the known positive correlation between isoprene emission and temperature (Singsaas et al., 1997) and the significantly enhanced growth rates of transgenic Arabidopsis under high temperature indicate that isoprene emission is the reason for the enhanced growth. Whether the enhanced growth is directly due to a protective role of isoprene or more likely due to a secondary effect of synthesized isoprene, i.e. isoprene production interfering with hormones such as foliar ABA, must be elucidated in future experiments. However, it has been recently observed that blocking the function of the MEP pathway with fosmidomycin has a reducing effect on ABA biosynthesis, leading to higher stomatal conductance in isoprene-emitting as well as in non-isoprene-emitting species (Barta and Loreto, 2006). A similar phenomenon could be in effect when redirecting the substrate synthesized by the MEP pathway, in this case to isoprene production.
Despite several studies showing that transgenic plants have retarded growth due to metabolic engineering of the MEP pathway (Fray et al., 1995; Aharoni et al., 2003, 2006), others have shown that in kudzu, for example, isoprene emission positively correlates not only with temperature but also with plant growth under high temperatures (Wiberley et al., 2005). In addition, isoprene emission capacity is achieved earlier under inducing conditions (temperature 35°C, light intensity 1,000 μmol m−2 s−1) in young kudzu plants (Wiberley et al., 2005) and delayed under cool temperatures in poplar (Populus tremuloides; Monson et al., 1994). While isoprene emission from plants naturally capable of this appears to play a significant role in plant survival, at least under stress conditions, our results with Arabidopsis indicate that isoprene protection against damage from transient thermal stress may not be widely applicable to isoprene nonemitters transformed to emit isoprene. Our photosynthetic gas-exchange studies showed no negative effect from transient but still moderate temperature stress in the wild type compared to transgenic isoprene-emitting Arabidopsis plants. Temperature stress created with rapid heat cycles does not have a negative effect on the recovery of net assimilation rate of the wild type, indicating that Arabidopsis does not need isoprene to protect against thermal stress. In fact, net assimilation of the highest isoprene-emitting line 9 seems to be more affected by the transient temperature stress than the wild type. A similar observation was shown for the non-isoprene-emitting leaves of Phaseolus vulgaris, which were more thermotolerant than isoprene-emitting oak (Quercus alba) and kudzu leaves (Singsaas et al., 1997).
On the other hand, comparable transient temperature stress experiments with transgenic poplars knocked down in isoprene emission clearly demonstrated a protective effect of isoprene emission on net assimilation and photosynthetic electron transport parameters in a natural isoprene emitter (Behnke et al., 2007). The lack of isoprene in fosmidomycin-fed reed (Phragmites australis) also reduced thermotolerance and restrained the recovery from high temperature stress (Velikova et al., 2005). Similarly, Sharkey et al. (2001) observed that fosmidomycin-fed natural isoprene emitters kudzu and oak (Quercus rubra) exposed to elevated temperatures and exogenous isoprene survived better during thermal stress than plants that received the same treatments without isoprene fumigation. However, with the molecular genetic approach, many of the potential side effects of fosmidomycin-feeding can be overcome, for example, the avoidance of inadvertently blocking other metabolic pathways such as those for hormones and carotenoids, as well as the intended drop in the generation of isoprenoids and the known but unwanted affects on ABA production. Similarly, exogenous isoprene fumigation that ignores the costs of isoprene production for the plant and does not affect the inside of the leaf tissues can be overcome. Our molecular genetic approach enables observations in non-isoprene-emitting species that would be difficult if not impossible to do without transformation, and our results further demonstrate the efficacy of such a method.
In summary, we have been able to demonstrate that overexpression of poplar ISPS in Arabidopsis resulted in isoprene-emitting Arabidopsis plants that show enhanced growth rates compared to the wild type under thermal stress. The fact that highest growth rates, higher DMADP levels, and enzyme activity were detected in young, developing plants indicates that enhanced growth of the transgenic plants under thermal stress is due to the introduced PcISPS gene. We have also shown dynamic measurements of photosynthetic gas exchange in Arabidopsis. According to these results, it seems that the altered phenotype of transgenic plants is not observed because isoprene would protect net assimilation from damage against thermal stress in this species as wild-type Arabidopsis is already well enough thermotolerant. However, before using this species as a model to study biological function of isoprene, care should be taken when considering several developmental and possibly environmental aspects that may affect metabolic flux through the MEP pathway in Arabidopsis. This species thought to be simple in metabolism may indeed not be so simple at all. This study paves the way to better understanding the possibilities and the limits these methods present when used to study isoprene biosynthesis. In particular, it emphasizes the potential difficulties that one may face when introducing an isoprenoid gene to a non-natural isoprene emitter.
MATERIALS AND METHODS
Plant Material and Plant Growth
Arabidopsis (Arabidopsis thaliana; ecotype Columbia-0) were cultivated in 7- × 7- × 8-cm plastic pots filled with fine turf:vermiculite:1.5 mm Quartzsand (8:1:1) and, for fertilization, Triabon and Osmocote (each 1 g L−1). Seeds were placed for 4 d at 4°C before being brought to growth chamber to germinate and to grow at 24°C:20°C (day:night), using a 16-h-light:8-h-dark photoperiod (PPFD 80 μmol photons m−2 s−1 during the light period). For thermal stress, 29°C:27°C (day:night) were used. Soil water content was maintained at a constant value by irrigating with tap water three times a week, and Hypoaspis miles (Re-natur GmbH) were occasionally spread on the soil to defend against herbivores.
For growth experiments involving leaf area measurement (performed in climate chambers of the Research Centre Jülich), plants were treated as described above with the following differences. Plants grew in ED73, a mixture of coarser turf, clay, and fertilizer (NPK 300/300/600 mg/L). Temperature in the growth chamber was 23°C:19°C and for thermal stress 29°C:24°C (day:night). Hypoaspis miles was not used.
Construction of Binary Vector for Overexpression of PcISPS in Arabidopsis
For cloning of Grey poplar (Populus x canescens) ISPS (EMBL AJ294819), a partial sequence from position 39 to position 1,868 from the original sequence was ligated into the bacterial expression vector pQE50 (Qiagen) by introducing a BamHI restriction site at the 5′ end and a KpnI restriction site at the 3′ end. This subclone, harboring the complete coding sequence of ISPS, was shown to produce isoprene when heterologously expressed in Escherichia coli (data not shown) and was therefore used for the further cloning steps.
The BamHI-KpnI fragment was ligated into the binary vector pBinAR (Höfgen and Willmitzer, 1990) under the control of a 35S promoter and an OCS terminator (Fig. 1A). Agrobacterium tumefaciens strain C58C1 pMP90 was transformed electrochemically using a Gene Pulser II (Bio-Rad), and clones were selected on LB-agar containing gentamicin (25 μg mL−1), rifampicin (100 μg mL−1), and kanamycin (25 μg mL−1).
Transformation of Arabidopsis and Screening of the Transgenic Lines
Arabidopsis plants were transformed using floral-dip technology (Clough and Bent, 1998), and F1 generation was screened and selected on Murashige and Skoog plates (1× Murashige and Skoog with Gamborg's vitamins [M 0404; Sigma], 1% [w/v] Suc, and 1% [w/v] phytagar [Invitrogen]) containing the selective agent kanamycin (50 μg mL−1).
RNA Isolation and cDNA Synthesis
Total RNA from leaves, roots, and inflorescences was isolated with the Qiagen RNeasy Minikit (Qiagen) following the Qiagen standard protocol. Amount and purity of isolated RNA were determined spectrophotometrically.
For first-strand cDNA synthesis, 3 μg of total RNA was reverse transcribed using oligo(dT) primers and Superscript II reverse transcriptase (Invitrogen) in a total volume of 20 μL according to manufacturer protocol. cDNA was stored at −20°C prior analysis.
Quantification of Transcript Levels by Quantitative Reverse Transcription-PCR
For quantitative PCR measurements of transcription rates of ISPS, the following primer set was used: forward 5′ ttt gcc tac ttt gcc gtg gtt caa aac 3′ and reverse 5′ tcc tca gaa atg cct ttt gta cgc atg 3′ (resulting in PCR segment length of 197 bp; Loivamäki et al., 2007). As a fluorescent marker for the increasing amount of double-stranded DNA, SYBR Green was used. The assays contained 12.5 μL of 2× SYBR Green PCR Master Mix (Applied Biosystems), 300 nm of each primer, and 5 μL of total cDNA (diluted five times) in a final volume of 25 μL. After a “hot start” (10 min, 95°C), 45 PCR cycles were performed with a 15-s melting step at 95°C and a 1-min annealing/extension step at 60°C on a GeneAmp 5700 sequence detection system (Applied Biosystems). For internal normalization, the transcript levels were related to the total RNA amount like described by Loivamäki et al. (2007).
Biochemical Analysis of ISPS Protein, Enzyme Activity, and Other Metabolic Intermediates (DMADP and Carotenoids)
For protein extraction, Arabidopsis leaves (approximately 300 mg) were suspended in 4 mL of plant extraction buffer (Mayrhofer et al., 2005), finely homogenized at 4°C using an ultra turrax and 2 mL of potter. Further steps as well as ISPS activity assay were performed as described by Mayrhofer et al. (2005), except that protein extracts from 2-week-old leaves (approximately 600 mg) were used and emitted isoprene was determined with proton transfer reaction (PTR)-mass spectrometry (MS) (see the description below).
Native PAGE (10% acrylamide) was performed on precast gels (Novex; Invitrogen) according to the manufacturer's protocol. From each sample, 20 μg of protein was loaded onto the gels. Protein transfer was achieved using a Millipore semidry electroblot system following the manufacturer's instructions. Immunoassaying of ISPS was performed according to Schnitzler et al. (2005). Alkaline phosphatase-conjugated secondary anti-rabbit antibody (Sigma-Aldrich) was detected with addition of 5-bromo-4-chloroindolyl-3-phosphate and nitro blue tetrazolium (10 mg of 5-bromo-4-chloroindolyl-3-phosphate and 5 mg of nitro blue tetrazolium in 20 mL of 1 m Tris-HCl, pH 8.8, 1 mm MgCl2). For determination of Mrs, prestained native protein standards (Serva) were used.
DMADP levels were determined as described by Brüggemann and Schnitzler (2002b) with following changes: 88% H3PO4 was added on single freeze-dried leaves for catalyzing the reaction from DMADP to isoprene and 4 m NaOH used for neutralization. Hydrolyzed isoprene was determined with PTR-MS.
Total carotenoid and chlorophyll levels were determined from transgenic lines (8 and 9) and wild type after Lichtenthaler and Wellburn (1983).
Headspace Collection for Isoprene Emission Analysis
For headspace analysis, weighed leaves, flowers, or roots (washed under tap water) from Arabidopsis were placed in 2-mL vials filled with 100 μL of mineral water and allowed to stabilize for 30 min on a light bench (PPFD approximately 300 μmol m−2 s−1 and air temperature approximately 35°C). After stabilization, another 150 μL of mineral water was added, and vials were sealed gas-tight and further incubated for 180 min. Before analysis of headspace with PTR-MS, vials were kept in darkness to interrupt the light-dependent isoprene formation.
Feeding with DOX
We enhanced artificially the flow through of the MEP pathway by feeding with 3 and 30 nm DOX, known to enter the chloroplast and be converted into DOXP (Wolfertz et al., 2003). Isoprene emission and DMADP level were determined as described above. The same leaves were used for both measurements, so that, after detection of isoprene with PTR-MS (standard method described below), the vials were opened and incubated again on the light bench for 120 min to increase the DMADP level again. The leaves were then removed and frozen rapidly with liquid N2 for DMADP measurements.
Analysis of Isoprene with PTR-MS
The functional screening on isoprene emission was performed with a newly developed headspace-analysis system using online PTR-MS, a combination of a proton transfer reaction drift tube and a quadrupole mass spectrometer. The instrument allows a fast detection of most VOCs in combination with low detection limits (10–100 pptv; for details see Lindinger et al., 1998; Schnitzler et al., 2004; Tholl et al., 2006). For transfer of the sample into the PTR-MS, the headspace of the vials was transferred into a 10-mL injection loop by flushing the vials with 10 mL of N2, and the samples were subsequently injected directly into the online MS with a high flow rate of 250 mL min−1. The system was calibrated by vials filled with calibration gas (10.9 ppm isoprene).
Determination of RGRs
For measuring RGR of Arabidopsis, two different methods were used: Either mass (experiments done in climate chambers of the Research Centre Karlsruhe) or leaf area (experiments done in climate chambers of the Research Centre Jülich) was measured. In both cases Arabidopsis seeds, homozygously expressing isoprene, were allowed to germinate as described above, but without selection, in soil.
In the experiments in which mass was measured to calculate RGR, seedlings were transferred to new pots after appearance of the two first leaves (five seedlings per pot; one in each corner and one in the middle). After 2 d of adaptation, temperature remained at 23°C or was switched to 29°C to create the desired growth conditions. Plants grew 7 d under these conditions before the start of the analysis. To determine RGR, FWs of 12 individual plants per line were measured every second day during 12 d. Plants were harvested such that the number of individuals in pots remained comparable.
For leaf area measurements, seeds were allowed to germinate as described above, but the plants were replanted to individual pots and allowed to grow for 14 d (normal conditions) or for 21 d (thermal stress) before onset of the measurement. A novel technique, the so-called GROWSCREEN setup (Walter et al., 2007), was used to measure projected total leaf areas and to determine their daily increments expressed as RGRs. Therefore, images of each seedling were acquired daily or every second day during 21 d, and the amount of pixels characterizing the leaf area was determined. Each pixel corresponded to a leaf area of 7,200 μm2. Color segmentation between green leaf area and brown/black background was performed on the basis of HLV-formatted images that were transformed from RGB (red, green, blue) images provided by the camera. For more details, see Gonzalez and Woods (2002) and Walter et al. (2007). RGR was calculated in both experiments analogously: RGR = 1/t × ln(AW2/AW1), where AW indicates either area or weight and t the time between the two measurements.
Measurement of Photosynthetic Gas Exchange and Isoprene Emission Rates from Arabidopsis Rosettes
New cuvettes suitable for Arabidopsis rosettes were developed to allow dynamic online monitoring of photosynthetic gas exchange and emission of VOCs, such as isoprene. The system consisted of four cuvettes (cuvette volume 530 mL) constructed of teflonized aluminum bodies covered with plastic glass lids (Fig. 5, A and B). Temperature inside the cuvette was regulated with Peltier elements and dynamically adjusted very quickly; +5°C in approximately 130 s and −5°C in approximately 100 s to a chosen leaf temperature measured by a thermocouple within the rosette. Light was provided by five LED lamps (DP3-W3-854; Osram) allowing light intensity to be increased up to a PPFD of 1,300 μmol photons m−2 s−1. Lamps and Peltier elements were cooled with cooling elements and ventilators (type 8414 NGH; Epm-Papst).
Clean air with 380 ppm CO2 was pushed with a flow of 2 L min−1 (2-L mass flow controllers; Bronkhorst) to all the cuvettes, in which air was circulated with small ventilators and pulled out to three-way valves (type NO-C-NC; Teocom). The three-way valves allowed the air to stream out either as waste or for gas analysis. In the latter case, the air stream was divided between fast isoprene sensor (FIS) drawing 650 mL min−1 (Hills Scientific; detailed description in Brüggemann and Schnitzler, 2002a) and LI-7000 CO2/H2O analyzer (LI-COR) drawing 600 mL min−1. The LI-7000 was calibrated daily with a CO2 gas standard (373 ppm) and the fast isoprene sensor weekly with 5.8 ppm isoprene in N2.
Changes in leaf temperature and switching between the valves (and cuvettes) were automated with a computer terminal. For the temperature-dependency studies (Fig. 6), measuring occurred in 5°C steps every 30 min from 30°C up to 45°C leaf temperature and returned similarly back to 30°C. The measurement was repeated two times from each of the four cuvettes in each temperature, 225 s from a cuvette at a time. Switching between the valves was automated with a computer terminal with a sequence that switched rapidly from one valve to the next one. Leaf temperature was adjusted with sliding starts of ±5°C per cycle (4 × 225 s) before beginning measurements from the cuvette in question.
For the temperature stress studies (Fig. 7), only one cuvette was used. After 20 min of stabilization time, six heat cycles (temperature switched every 10 min from 30°C to 40°C and back) were performed. After the heat cycles, plants were allowed to recover at a leaf temperature of 30°C for 20 min.
Stomatal conductance was calculated after Von Caemmerer and Farquhar (1981) and Ball (1987).
Statistical Analysis
Statistical and correlation analysis was performed with SPSS for Windows NT (Release 8.0) and Sigmaplot 2000 for Windows (Version 6.10), both programs from SPSS Inc.
Sequence data from this article can be found in the GenBank/EMBL data libraries under accession number AJ294819.
Acknowledgments
We greatly acknowledge the provision of DOX by Prof. T.N. Rosenstiel (University of Portland, Oregon) and of an AtACT2 fragment of Arabidopsis by Prof. U.I. Flügge (University of Cologne, Germany). We are also grateful to Dr. S. Louis (Research Centre Karlsruhe, Germany) for the critical reading of the manuscript and to T. Winters (Australian National University, Australia) who kindly proofed the English style and spelling of the manuscript.
The work was supported by the European Commission in the frame of the Marie Curie Research Training Network ISONET.
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: Jörg-Peter Schnitzler (joerg-peter.schnitzler@imk.fzk.de).
Open Access articles can be viewed online without a subscription.
References
- Aharoni A, Giri AP, Deuerlein S, Griepink F, de Kogel WJ, Verstappen FWA, Verhoeven HA, Jongsma MA, Schwab W, Bouwmeester HJ (2003) Terpenoid metabolism in wild-type and transgenic Arabidopsis plants. Plant Cell 15 2866–2884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aharoni A, Jonsma MA, Kim TY, Ri MB, Giri AP, Verstappen FWA, Schwab W, Brouwmeester HJ (2006) Metabolic engineering of terpenoid biosynthesis in plants. Phytochem Rev 5 49–58 [Google Scholar]
- Aubourg S, Lecharny A, Bohlmann J (2002) Genomic analysis of the terpenoid synthase (AtTPS) gene family of Arabidopsis thaliana. Mol Genet Genomics 267 730–745 [DOI] [PubMed] [Google Scholar]
- Ball JT (1987) Calculations related to gas exchange. In E Zeiger, GD Farquhar, JR Ciwan, eds, Stomatal Function. Stanford University Press, Stanford, CA, pp 381–390
- Barta C, Loreto F (2006) The relationship between the methyl-erythritol phosphate pathway leading to emission of volatile isoprenoids and abscisic acid content in leaves. Plant Physiol 141 1676–1683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behnke K, Ehlting B, Teuber M, Bauerfeind M, Louis S, Hänsch R, Polle A, Bohlmann J, Schnitzler JP (2007) Transgenic, non-isoprene-emitting poplars don't like it hot. Plant J doi/10.1111/j.1365–313X.2007.03157.x [DOI] [PubMed]
- Biesenthal TA, Wu Q, Shepson PB, Wiebe HA, Anlauf KG, MacKay GI (1997) A study of relationships between isoprene, its oxidation products, and ozone, in the lower Fraser valley, BC. Atmos Environ 31 2049–2058 [Google Scholar]
- Botella-Pavia P, Besumbes O, Phillips MA, Carretero-Paulet L, Boronat A, Rodriquez-Conception M (2004) Regulation of carotenoid biosynthesis in plants: evidence for a key role of hydroxymethylbutenyl diphosphate reductase in controlling the supply of plastidial isoprenoid precursors. Plant J 40 188–199 [DOI] [PubMed] [Google Scholar]
- Brüggemann N, Schnitzler JP (2002. a) Comparison of isoprene emission, intercellular isoprene concentration and photosynthetic performance in water-limited oak (Quercus pubescens Willd. and Quercus robur L.) saplings. Plant Biol 4 456–463 [Google Scholar]
- Brüggemann N, Schnitzler J-P (2002. b) Diurnal variation of dimethylallyl diphosphate concentrations in oak (Quercus robur L.) leaves. Physiol Plant 115 190–196 [DOI] [PubMed] [Google Scholar]
- Carretero-Paulet L, Ahumada I, Cunillera N, Rodriguez-Conception M, Ferrer A, Boronat A, Campos N (2002) Expression and molecular analysis of the Arabidopsis DXR gene encoding 1-deoxy-D-xylulose 5-phosphate reductoisomerase, the first committed enzyme of the 2-C-methyl-D-erythritol 4-phosphate pathway. Plant Physiol 129 1581–1591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carretero-Paulet L, Cairó A, Botella-Pavía P, Besumbes O, Campos N, Boronat A, Rodriquez-Conceptión M (2006) Enhanced flux through the methylerythritol 4-phosphate pathway in Arabidopsis plants overexpressing deoxyxylulose 5-phosphate reductoisomerase. Plant Mol Biol 62 683–695 [DOI] [PubMed] [Google Scholar]
- Chen F, Ro DK, Petri J, Gershenzon J, Böhlmann J, Pichersky E, Tholl D (2004) Characterization of a root-specific Arabidopsis terpene synthase responsible for the formation of the volatile monoterpene 1,8-cineole. Plant Physiol 135 1956–1966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen F, Tholl D, D'Auria JC, Farooq A, Pichersky E, Gershenzon J (2003) Biosynthesis and emission of terpenoids volatiles from Arabidopsis flowers. Plant Cell 15 481–494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clough SJ, Bent AF (1998) Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J 16 735–743 [DOI] [PubMed] [Google Scholar]
- Derwent RG, Jenkin ME, Saunders SM, Pilling MJ (1998) Photochemical ozone creation potentials for organic compounds in northwest Europe calculated with a master chemical mechanism. Atmos Environ 32 2429–2441 [Google Scholar]
- Enfissi EMA, Fraser PD, Lois LM, Boronat A, Schuch W, Bramley BM (2005) Metabolic engineering of the mevalonate and non-mevalonate isopentenyl diphosphate forming pathways for the production of health promoting isoprenoids in tomato. Plant Biotechnol J 3 17–27 [DOI] [PubMed] [Google Scholar]
- Eisenreich W, Rohdich F, Bacher A (2001) Deoxyxylulose phosphate pathway of terpenoids. Trends Plant Sci 6 78–84 [DOI] [PubMed] [Google Scholar]
- Estevez JM, Cantero A, Reindl A, Reichler S, Leon P (2001) 1-Deoxy-D-xylulose 5-phosphatase, a rate limiting enzyme for plastidic isoprenoid biosynthesis in plants. J Biol Chem 276 22901–22909 [DOI] [PubMed] [Google Scholar]
- Fray RG, Wallace A, Fraser PD, Valero D, Hedden P, Bramley PM, Grierson D (1995) Constitutive expression of fruit phytoene synthase in transgenic tomatoes causes dwarfism by redirection of metabolites from the gibberellin pathway. Plant J 89 1351–1357 [Google Scholar]
- Höfgen R, Willmitzer L (1990) Biochemical and genetic analysis of different patatin isoforms expressed in various organs of potato (Solanum tuberosum). Plant Sci 66 221–230 [Google Scholar]
- Gonzalez R, Woods R (2002) Digital Image Processing, Ed 2. Prentice Hall International, Upper Saddle River, NJ
- Kesselmeier J, Staudt M (1999) Biogenic volatile organic compounds: An overview on emission, physiology and ecology. J Atmos Chem 33 23–88 [Google Scholar]
- Lavy M, Zuker A, Larkov O, Ravid U, Lewinsohn E, Vainstein A, Weiss D (2002) Linalool and linalool oxide production in transgenic carnation flowers expressing the Clarkia breweri linalool-synthase gene. Mol Breed 9 103–111 [Google Scholar]
- Lewinsohn E, Schalechet F, Wilkinson J, Matsui K, Tadmor Y, Nam KH, Amar O, Lastochkin E, Larkov O, Ravid U, et al (2001) Enhanced levels of the aroma and flavor compound S-linalool by metabolic engineering of the terpenoid pathway in tomato fruits. Plant Physiol 127 1256–1265 [PMC free article] [PubMed] [Google Scholar]
- Lichtenthaler HK (1999) The 1-deoxy-D-xylulose-5-phosphate pathway of isoprenoid biosynthesis in plants. Annu Rev Plant Physiol Plant Mol Biol 50 47–65 [DOI] [PubMed] [Google Scholar]
- Lichtenthaler HK, Wellburn AR (1983) Determination of total carotenoids and chlorophyll a and b of leaf extracts in different solvents. Biochem Soc Trans 603 591–592 [Google Scholar]
- Lim HJ, Carlton AG, Turbin BJ (2005) Isoprene forms secondary organic aerosol through cloud processing: model simulations. Environ Sci Technol 15 4441–4446 [DOI] [PubMed] [Google Scholar]
- Lindinger W, Hansel A, Jordan A (1998) Proton-transfer-reaction mass spectrometry (PTR-MS): on-line monitoring of volatile organic compounds at pptv levels. Chem Soc Rev 27 347–354 [Google Scholar]
- Logan BA, Monson RK, Potosnak MJ (2000) Biochemistry and physiology of foliar isoprene production. Trends Plant Sci 5 477–481 [DOI] [PubMed] [Google Scholar]
- Loivamäki M, Louis S, Cinege G, Zimmer I, Fischbach RJ, Schnitzler JP (2007) Circadian rhythms of isoprene biosynthesis in grey poplar leaves. Plant Physiol 143 540–551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loreto F, Mannozzi M, Maris C, Nascetti P, Ferranti F, Pasqualini S (2001) Ozone quenching properties of isoprene and its antioxidant role in leaves. Plant Physiol 126 993–1000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loreto F, Velikova V (2001) Isoprene produced by leaves protects the photosynthetic apparatus against ozone damage, quenches ozone products, and reduces lipid peroxidation of cellular membranes. Plant Physiol 127 1781–1787 [PMC free article] [PubMed] [Google Scholar]
- Lücker J, Bouwmeester HJ, Schwab W, Blaas J, van der Plas LHW, Verhoeven HA (2001) Expression of Clarkia S-linalool synthase in transgenic petunia plants results in the accumulation of S-linalyl-β-D-glucopyranoside. Plant J 27 315–324 [DOI] [PubMed] [Google Scholar]
- Lücker J, Schwab W, Franssen MCR, van der Plas LHW, Bouwmeester HJ, Verhoeven HJ (2004. a) Metabolic engineering of monoterpene biosynthesis: two step production of (+)-trans-isopiperitenol by tobacco. Plant J 39 135–145 [DOI] [PubMed] [Google Scholar]
- Lücker J, Schwab W, van Hautum B, Blaas J, van der Plas LHW, Bouwmeester HJ, Verhoeven HA (2004. b) Increased and altered fragrance of tobacco plants after metabolic engineering using three monoterpene synthases from lemon. Plant Physiol 14 510–519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magel E, Mayrhofer S, Müller A, Zimmer I, Hampp R, Schnitzler JP (2006) Determination of the role of products of photosynthesis in substrate supply of isoprenoid biosynthesis in poplar leaves. Atmos Environ 40 S138–S151 [Google Scholar]
- Mahmoud SS, Croteau RB (2001) Metabolic engineering of essential oil yield and composition in mint by altering expression of deoxyxylulose phosphate reductoisomerase and menthofuran synthase. Proc Natl Acad Sci USA 98 8915–8920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayrhofer S, Teuber M, Zimmer I, Louis S, Fischbach RJ, Schnitzler JP (2005) Diurnal and seasonal variation of isoprene biosynthesis-related genes in grey poplar leaves. Plant Physiol 139 474–484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller B, Oschinski C, Zimmer W (2001) First isolation of an isoprene synthase gene from poplar and successful expression of the gene in Escherichia coli. Planta 213 483–487 [DOI] [PubMed] [Google Scholar]
- Monson RK, Harley PC, Litvak ME, Wildermuth M, Guenther AB, Zimmerman PR, Fall R (1994) Environmental and developmental controls over the seasonal pattern of isoprene emission from aspen leaves. Oecologia 99 260–270 [DOI] [PubMed] [Google Scholar]
- Pichersky E, Gershenzon J (2002) The formation and function of plant volatiles: parfumes for pollinator attraction and defence. Curr Opin Plant Biol 5 237–243 [DOI] [PubMed] [Google Scholar]
- Rodriguez-Conception M, Boronat A (2002) Elucidation of the methylerythritol phosphate pathway for isoprenoid biosynthesis in bacteria and plastids. A metabolic milestone achieved through genomics. Plant Physiol 130 1079–1089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohmer M, Knanin M, Simonin P, Sutter P, Sahm H (1993) Isoprenoid biosynthesis in bacteria: a novel pathway for the early steps leading to isopentenyl diphosphate. Biochem J 295 517–524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenstiel TN, Ebbets AL, Khatri WC, Fall R, Monson RK (2004) Induction of poplar leaf nitrate reductase: a test of extrachloroplastic control of isoprene emission rate. Plant Biol 6 12–21 [DOI] [PubMed] [Google Scholar]
- Sanadze JA (1957) Emission of organic matters by leaves of Robinia pseudoacacia L. Soobshch Akad Nauk Gruz SSR 19 83–86 [Google Scholar]
- Sasaki K, Ohara K, Yazaki K (2005) Gene expression and characterization of isoprene synthase from Populus alba. FEBS Lett 579 129–134 [DOI] [PubMed] [Google Scholar]
- Schnitzler JP, Gras M, Kreuzwieser J, Heizmann U, Rennenberg H, Wisthaler A, Hansel A (2004) Contribution of different carbon sources to isoprene biosynthesis in poplar leaves. Plant Physiol 135 152–160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnitzler JP, Zimmer I, Bachl A, Arend M, Fromm J, Fischbach RJ (2005) Biochemical properties of isoprene synthase from poplar (Populus x canescens). Planta 222 777–786 [DOI] [PubMed] [Google Scholar]
- Sharkey TD, Chen X, Yeh S (2001) Isoprene increases thermotolerance of fosmidomycin-fed leaves. Plant Physiol 125 2001–2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharkey TD, Singsaas EL (1995) Why plants emit isoprene. Nature 374 769 [Google Scholar]
- Sharkey TD, Yeh S (2001) Isoprene emission from plants. Annu Rev Plant Physiol Plant Mol Biol 52 407–436 [DOI] [PubMed] [Google Scholar]
- Sharkey TD, Yeh S, Wiberley AE, Falber TG, Gong D, Fernandez DE (2005) Evolution of the isoprene biosynthetic pathway in kudzu. Plant Physiol 137 700–712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singsaas EL, Lerdau M, Winter K, Sharkey TD (1997) Isoprene increases thermotolerance of isoprene-emitting species. Plant Physiol 115 1413–1420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singsaas EL, Sharkey TD (1998) The regulation of isoprene emission responses to rapid leaf temperature fluctuations. Plant Cell Environ 21 1181–1188 [Google Scholar]
- Steeghs M, Bais HP, de Grouw J, Goldan P, Kluster W, Northway M, Fall R, Vivanco JM (2004) Proton transfer-reaction mass spectrometry as a new tool for real time analysis of root-secreted volatile organic compounds in Arabidopsis. Plant Physiol 135 47–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tholl D, Boland W, Hansel A, Loreto F, Röse USR, Schnitzler JP (2006) Practical approaches to plant volatile analysis. Plant J 45 540–560 [DOI] [PubMed] [Google Scholar]
- Tholl D, Chen F, Petri J, Gershenzon J, Pichersky E (2005) Two sesquiterpene synthases are responsible for the complex mixture of sesquiterpenes emitted from Arabidopsis flowers. Plant J 42 757–771 [DOI] [PubMed] [Google Scholar]
- Thompson AM (1992) The oxidizing capacity of the Earth's atmosphere: probable past and future changes. Science 256 1157–1165 [DOI] [PubMed] [Google Scholar]
- Van Poecke RMP, Posthumus MA, Dicke M (2001) Herbivore induced volatile production by Arabidopsis thaliana leads to attraction of the parasitoid Cotesia rubecula: chemical, behavioural and gene-expression analysis. J Chem Ecol 27 1911–1928 [DOI] [PubMed] [Google Scholar]
- Velikova V, Loreto F (2005) On the relationship between isoprene emission and thermotolerance in Phragmites australis leaves exposed to high temperatures and during the recovery from a heat stress. Plant Cell Environ 28 318–327 [Google Scholar]
- Velikova V, Pinelli P, Loreto F (2005) Consequences of inhibition of isoprene synthesis in Phragmites australis leaves exposed to elevated temperatures. Agric Ecosyst Environ 106 209–217 [Google Scholar]
- Von Caemmerer S, Farquhar GD (1981) Some relationships between the biochemistry of photosynthesis and the gas exchange of leaves. Planta 153 376–387 [DOI] [PubMed] [Google Scholar]
- Walter A, Scharr H, Gilmer F, Zieger R, Nagel KA, Ernst M, Wiese A, Virnich O, Christ MM, Uhlig B, et al (2007) Dynamics of seedling growth acclimation towards altered light conditions can be quantified via GROWSCREEN: a setup and procedure designed for rapid optical phenotyping of different plant species. New Phytol 174 447–455 [DOI] [PubMed] [Google Scholar]
- Wiberley AE, Linskey AR, Falbel TG, Sharkey TD (2005) Development of the capacity for isoprene emission in Kudzu. Plant Cell Environ 28 898–905 [Google Scholar]
- Wolfertz M, Sharkey TD, Boland W, Kühnemann F, Yeh S, Weise SE (2003) Biochemical regulation of isoprene emission. Plant Cell Environ 26 1357–1364 [Google Scholar]