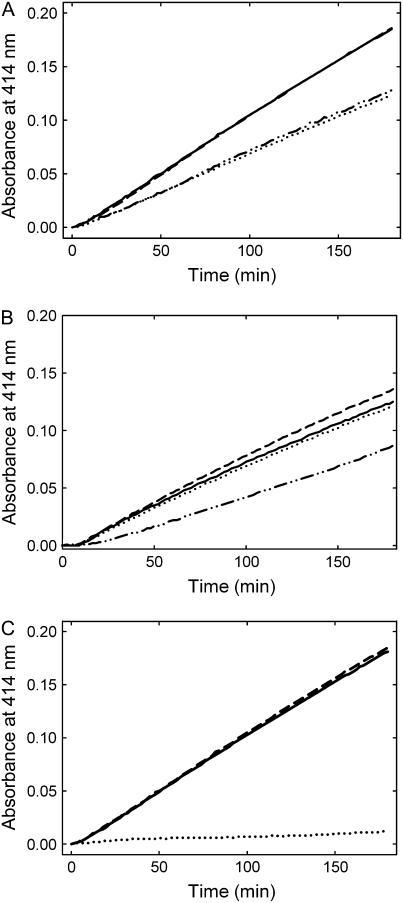
Figure 4.
Enzymatic formation of PLP. A, Initial rates of activity observed in the presence of R5P (0.5 mm) and either dl-G3P (1 mm) or DHAP (0.5 mm; solid and dashed line, respectively), or Ru5P (0.5 mm) and either dl-G3P (1 mm) or DHAP (0.5 mm; dotted and dashed/dotted line, respectively). All assays shown were carried out in the presence of 20 mm Gln and 10 μm each of PDX1.1 and PDX2. B, Initial rates observed as a function of the triose sugar in the presence of ammonium sulfate as the N source; R5P and dl-G3P in the presence or absence of PDX2 (solid and dashed line, respectively) or R5P and DHAP (dotted and dashed/dotted line, respectively). PDX1.1 was present under all conditions shown. C, Comparison of PLP synthase activity employing all three PDX1 homologs in the presence of PDX2; PDX1.1 (solid line), PDX1.2 (dotted line), and PDX1.3 (dashed line). The assays were performed using R5P, dl-G3P, and Gln. The concentrations of each component in B and C were as described in A.