Abstract
Aim
In paraneoplastic neurological syndromes (PNS) associated with small cell lung cancer (SCLC) and Hu antibodies (Hu-PNS), Hu antigens expressed by the tumour hypothetically trigger an immune response that also reacts with Hu antigens in the nervous system, resulting in tumour suppression and neuronal damage. To gain more insight into the hypothesized CD8+ T cell-mediated immune pathogenesis of these syndromes, we searched for circulating HuD-specific CD8+ T cells in a large cohort of Hu-PNS patients and controls.
Patients and methods
Blood was tested from 43 Hu-PNS patients, 31 Hu antibody negative SCLC patients without PNS and 54 healthy controls. Peripheral blood mononuclear cells (PBMC) were stimulated with HuD protein-spanning peptide pools (15-mers) and individual HuD-derived peptides (9-mers) and analysed by cytokine flow cytometry and interferon-γ ELISPOT-assays. Additionally, HuD-based Class I HLA multimers were used to visualize HuD-specific CD8+ T cells.
Results
No HuD-specific CD8+ T cells could be detected in the blood of Hu-PNS patients or controls.
Conclusions
Our results do not support a role for HuD-specific CD8+ T cells in Hu-PNS. Further studies should focus on the detection of circulating HuD-specific CD4+ T cells and examine the antigen specificity of T cells in affected tissues.
Keywords: Paraneoplastic, Anti-tumour immunity, T lymphocyte, SCLC, HuD, HLA class I multimer
Introduction
Paraneoplastic neurological syndromes (PNS) are considered as naturally occurring, successful anti-tumour immune responses in humans [6]. However, this tumour immunity goes along with autoaggression against the nervous system, resulting in severe neurological dysfunction [9]. The mechanisms responsible for the anti-tumour response and neuronal damage are poorly understood. Antigens expressed by the tumour that are normally restricted to neurons (so-called onconeuronal antigens) hypothetically trigger an immune response that cross-reacts with the same antigens in the nervous system [7]. One of the most frequently involved tumours is small cell lung cancer (SCLC) and approximately 50% of patients with PNS and SCLC have high-titre Hu antibodies (Hu-PNS). Hu antibodies are directed against a family of neuron specific, mRNA binding proteins, of which HuD is the best-documented member. Consistent expression of HuD in all SCLC suggests that HuD plays a central role in triggering the immune response [15, 17].
High titres of Hu antibodies in serum and cerebrospinal fluid suggested a pathogenic role for these antibodies, that could, however, never be proven in animal models [3, 22]. Furthermore, expression of Hu antigens is exclusively intracellular, and it is therefore difficult to understand how such antibodies could target tumours or neurons [5]. In addition, pathological examination of PNS neuronal tissue demonstrated localized inflammatory cell infiltrates, containing B cells, CD4+ and CD8+ T cells, in the proximity of overt neuronal cell damage [2, 13, 18, 25]. The presence of an oligoclonal CD8+ T cell infiltrate in nervous tissues and tumours of Hu-PNS patients further suggests an immunopathogenic role for such cells [18, 25].
Some authors report the presence of HuD peptide-specific CD8+ T cells in the blood of these patients, but also in that of apparently healthy controls (HC) [19, 20, 24]. In addition, the presence of circulating HuD-specific CD4+ T cells has been suggested [1].
Here, we have investigated the presence of circulating HuD-specific T cells in a large cohort of Hu-PNS patients and controls. Despite a multifaceted approach mainly geared towards the detection of HuD-reactive CD8+ T cells and, to a somewhat lesser extent, CD4+ T cells, no such cells were detected in the blood of Hu-PNS patients and controls.
Materials and methods
Patients
Forty-three patients with high-titred Hu antibodies and a definite clinical diagnosis of PNS [12], 31 Hu antibody negative SCLC patients without neurological symptoms or signs (SCLC) and 54 apparently HC were tested. The Erasmus MC Institutional Review Board approved the study and all individuals provided written informed consent. The individuals’ class I HLA alleles were typed by standard diagnostic PCR at the two-digit resolution level. Patient characteristics are shown in the Table 1. Anti-Hu IgG titres were determined as described previously [21] and in Hu-PNS the dependence in activities of daily living was scored using the modified Rankin scale [21]. Twenty-five HC were male and 29 female, their median age was 46 years (range 17–89) and all were Hu antibody negative. No HC had received previous chemotherapy or immunosuppressive treatment and 30 HC (56%) were CMV-seropositive.
Table 1.
Patient characteristics at the time of study entry
| Hu-PNS | SCLC | |
|---|---|---|
| N | 43 | 31 |
| Age (median, range) | 64 (4–81)a | 61 (40–83) |
| Gender (M/F) | 15/28 | 21/10 |
| Hu-Ab titre (median, range) | 12,800 (400–204,800) | Negative |
| CMV serostatus (pos/neg) | 29/14 | 17/14 |
| Paraneoplastic neurological syndrome | NA | |
| PSN | 27 | |
| PEM | 5 | |
| PCD | 4 | |
| PLE/ BE | 3 | |
| Pseudo-obstruction | 2 | |
| Motor neuron disease | 2 | |
| Tumour | ||
| No tumour | 8b | 0 |
| SCLC | 31 | 31 |
| Limited | 28 | 17 |
| Extended | 3 | 14 |
| NSCLC | 2 | 0 |
| Prostate | 1 | 0 |
| Neuroblastoma | 1 | 0 |
| Prior treatment | ||
| None | 27 | 27 |
| Chemotherapy ± immunosuppression | 16 | 4 |
| Neurological symptoms | NA | |
| Interval onset symptoms (study entry) | 5 months (2–15)c | |
| Interval onset symptoms (diagnosis) | 4 months (1–12)c | |
| Progressive at study entryd | 34 (79%) | |
| Modified Rankin score | NA | |
| MRS = 2 | 7 | |
| MRS = 3 | 22 | |
| MRS = 4 | 10 | |
| MRS = 5 | 4 | |
Hu-Ab Hu antibody, CMV cytomegalovirus, pos positive, neg negative, NA not applicable, PSN paraneoplastic sensory neuronopathy, PEM paraneoplastic encephalomyelitis, PCD paraneoplastic cerebellar degeneration, PLE paraneoplastic limbic encephalitis, BE brainstem encephalitis, SCLC small cell lung cancer, NSCLC non-small cell lung cancer, MRS modified Rankin score
a One Hu-PNS patient was a 4-year-old boy with an underlying neuroblastoma. The remaining Hu-PNS patients were aged between 49 and 81 years
b No tumour mass visible on CT-scan or FDG-PET scan
c Median (ranges) of intervals are shown
d Progression of neurological symptoms was defined by the increase of at least one point on the modified Rankin scale during 2 months prior to study entry
Reagents
Ninety-three HuD protein-spanning synthetic peptides, 15-mers with 11 amino acids overlap, were pooled to constitute the HuD peptide mix (HuDmix) and smaller peptide pools [Jerini Peptide Technologies (JPT), Berlin, Germany]. For interferon-γ enzyme-linked immunosorbent spot-forming (IFN-γ ELISPOT) assays and the construction of HLA class-I multimers, HuD-derived 9- and 10-mers were selected based on previous studies [19, 20]. The phycoerythrin (PE)-labeled multimers and corresponding peptides used were: HLA-A*0101-147ELEQLFSQY155, HLA-A*0101-245RLDNLLNMAY254, HLA-A*0201-86SLGYGFVNYI95, HLA-A*0201-248NLLNMAYGV256, HLA-A*0201-315QLFGPFGAV323, HLA-A*0201-362RLGDRVLQV370, and HLA-A*2402-154QYGRIITSRI163 (ProImmune, Oxford, UK). As positive and negative controls, HLA-A*0201-495NLVPMVATV503 [CMV phosphoprotein-65 (CMV-pp65); Beckman Coulter, San Diego, CA] and HLA-A*0201 presenting an irrelevant peptide (ProImmune), respectively, were included.
To measure general T-cell responsiveness, we used phorbol myristate acetate (PMA) plus ionomycin, or phytohemagglutinin (PHA). A peptide pool containing 15-mers spanning CMV-pp65 (JPT) was used as positive- and negative antigen-specific control in CMV seropositive and seronegative individuals, respectively.
Cytokine flow cytometry
Peripheral blood mononuclear cells (PBMC) were isolated within 12 h after venipuncture and stimulated in duplicate as described elsewhere [14]. Briefly, 2 × 106 PBMC were incubated at 37°C in a CO2 incubator for 18 h with 1 μg/ml HuDmix, 1 μg/ml CMV-pp65, 1 μg/ml ionomycin plus 25 ng/ml PMA, or without antigen. After 2 h of stimulation, brefeldin A was added to one of the duplicate tubes allowing for intracellular accumulation of cytokines in activated T cells. Brefeldin A was not added to the second tube to allow detection of secreted cytokines in the supernatant. In some individuals, additional stimulation was performed in the presence of co-stimulatory monoclonal antibodies (mAb) directed against CD28 and CD49d (BD Biosciences, San Jose, CA). Stimulated PBMC were stained and analysed using anti-CD3 conjugated with peridinyl chlorophyllin (PerCP), anti-CD8 conjugated with allophycocyanin (APC), anti-interferon (IFN)-γ conjugated with fluorescein isothiocyanate (FITC), anti-tumour necrosis factor (TNF)-α conjugated with PE, or appropriate isotype control mAb (all from BD Biosciences) [14]. CD4+ T cells were defined as CD3+, 8−. Positive responses were defined by (1) percentage of cytokine-positive CD4+ or CD8+ T cells >2 times the negative control (i.e., no antigen) and (2) ≥0.1% of the total number of CD4+ or CD8+ T cells, each after subtraction of isotype control results.
Detection of secreted cytokines
The secretion levels of IFN-γ, TNF-α, interleukin (IL)-2, IL-4, IL-5 and IL-10 were measured in supernatants using a cytometric bead array (BD Biosciences). Based on CMV data (not shown), a positive result was defined as cytokine concentration >2 times the background (no antigen) and a minimum level of 50 pg/ml.
IFN-γ ELISPOT
PBMC (2 × 105/well) were pre-stimulated in duplicate in 96-well plates with culture medium containing 3 μg/ml HuD 9-mers, HuDmix, CMV-pp65, PHA, or no antigen for 1.5 h at 37°C and 5% CO2 [10]. The PBMC were subsequently transferred to anti-IFN-γ-coated ELISPOT plates (Nalge Nunc, Rochester, NY) for a further 18-h incubation. The ELISPOT assay was performed using standard protocols and automated reading (AELVIS GmbH, Hanover, Germany) [10]. The mean number of spot-forming cells (SFC) in duplicate wells was used as assay outcome. Positive results were defined by numbers of SFC/well >3 times background (no antigen) and a minimum of 15 SFC/well.
Detection of HuD-specific CD8+ T cells using Class-I HLA multimers
Thawed PBMC were stained as described previously [11]. Following acquisition of 1 × 105 viable (i.e., 7-amino-actinomycin-D [7AAD] negative) CD8+ T cells, a positive result required a percentage of ≥0.1% of viable CD8+ T cells binding the HuD multimer and a brightly staining HuD multimer-binding CD8+ T-cell population that did not overlap with the dimly staining irrelevant multimer-binding T-cell population.
Results
Cytokine flow cytometry of PBMC after stimulation with HuD-derived peptides
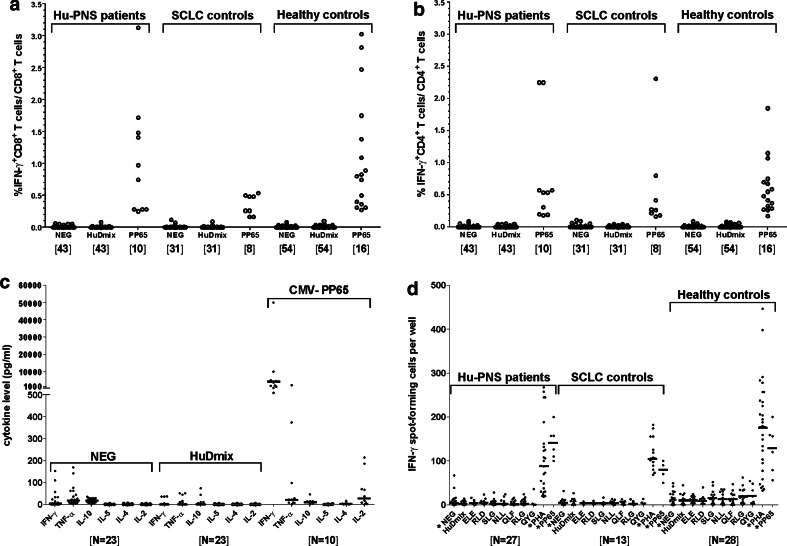
Stimulation with PMA and ionomycin induced IFN-γ production in both CD4+ and CD8+ T cells of all individuals (not shown). Whilst all CMV-seropositive individuals specifically responded to CMV-pp65, no HuD-specific T-cell reactivity was observed in any of the Hu-PNS patients, SCLC patients or HC (Fig. 1a, b). Similar results were obtained when intracellular TNF-α (not shown) or secreted cytokines (Fig. 1c) were measured. The use of co-stimulatory antibodies in combination with HuDmix did not result in the detection of HuD-specific T-cell reactivity (not shown).
Fig. 1.
Cytokine production in response to HuDmix and HLA-matched HuD 9-mers. Proportions of CD8+ (panel a) and CD4+ (panel b) T cells expressing intracellular IFN-γ after stimulation with HuDmix in Hu-PNS patients, SCLC and healthy controls. Each dot represents the result observed in a single individual (panel c). After stimulation of PBMC with HuDmix and control antigens, the indicated cytokines were measured in assay supernatants. The results are shown for Hu-PNS patients only. Horizontal lines indicate median values of each group (panel d). ELISPOT assay showing the number of IFN-γ SFC after stimulation of 2 × 105 PBMC with HuDmix or HuD 9-mers. Each dot represents the mean result of duplicates for each stimulus in each individual (panels a–d). Responses to CMV antigens are shown for CMV seropositive individuals only, as they were consistently negative in CMV seronegative individuals (not shown). The numbers of individuals tested are given in between brackets. NEG negative control (incubation without antigen), HuDmix HuD protein-spanning peptide pool, PP65 CMV pp-65 protein-spanning peptide pool, IL interleukin TNF tumour necrosis factor, IFN interferon, SFC spot-forming cell, PHA phytohemagglutinin. ELE, RLD, SLG, NLL, QLF, RLG and QYG designate individual HuD-based peptides
IFNγ-ELISPOT assay on PBMC stimulated with HuD-derived 9-mers
All individuals responded to PHA as determined by IFNγ-ELISPOT assay. In addition, all CMV-seropositive individuals responded to CMV-pp65. However, no T cell reactivity towards the previously described class-I HLA-binding HuD peptides [20] was detected in individuals with the appropriate HLA types in any of the study groups (Fig. 1d).
Analysis of HuD-specific CD8+ T cells using Class-I HLA multimers
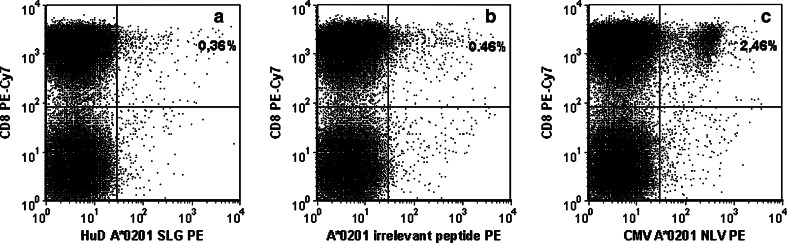
Finally, we investigated the presence of HuD-specific CD8+ T cells in PBMC using Class-I HLA multimers containing HuD-derived peptides. Whilst CMV-seropositive individuals with the appropriate HLA types showed distinct populations of pp65 multimer-binding CD8+ T cells, no HuD-specific CD8+ T cells were observed in individuals with the appropriate HLA types in any of the study groups (Fig. 2).
Fig. 2.
Analysis of HuD peptide-loaded, Class-I HLA multimer-binding CD8+ T cells. PBMC from a CMV-seropositive Hu-PNS patient were stained with HLA-A*0201 multimers loaded with the HuD peptide SLGYGFVNYI (panel a), an irrelevant peptide (panel b) or with the CMV-pp65 peptide NLVPMVATV (panel c). All data shown are obtained after selection of T cells (i.e., CD3+, low sideward scatter signals) [12]. Binding of Class-I HLA multimers (panels a–c, horizontal axes) was analysed in relation to CD8 expression. In this example, the proportion of SLGYGFVNYI multimer-binding CD8+ T cells (0.36%) was similar to that of irrelevant multimer-binding CD8+ T cells (0.46%); binding resulted in low-intensity fluorescence signals only. In contrast, 2.46% of CD8+ T cells bound the NLVPMVATV multimer resulting in high-intensity fluorescence signals from most CD8+ T cells
Discussion
We set out to detect HuD-specific T cells in the blood of Hu-PNS patients as they are postulated to play a pivotal role in the immunopathology of this disease. Although we applied three different approaches we could not detect circulating HuD-specific T cells.
First, to induce cytokine responses in T cells, we used 15-mer protein-spanning peptide pools that have the advantage of covering the full protein sequence and of eliciting both CD8+ and CD4+ T-cell responses [14], as demonstrated by the CD8+ and CD4+ CMV-pp65-specific T-cell responses. However, no CD8+ or CD4+ HuD-specific T-cell responses were observed. These results are at variance with the detection of HuD-specific CD4+ T-cell proliferative responses in PBMC of Hu-PNS patients by Benyahia et al. [1]. This discrepancy may be explained by differences in read-out (i.e., 3-day lymphocyte proliferation in Benyahia’s study [1] vs. overnight cytokine production in ours) and the use of recombinant HuD protein [1] versus a protein-spanning 15-mer peptide pool (this manuscript).
We then studied responses to HLA class-I binding HuD peptides that were previously selected [20]. Using the same experimental setup and HuD 9-mer peptides, Rousseau et al. [20] reported HuD-specific T-cell reactivity in 7/10 Hu-PNS patients and in 3/10 HC [20]. In that small study a positive response was defined as an experimental value ≥2 times above background. With that criterion, 3 PNS, 2 SCLC and 4 HC would have been classified as HuD T-cell responders in our study. However, using that cut-off we would also have detected T-cell reactivity in individuals whose Class-I HLA molecules did not have the appropriate binding motifs (data not shown). Therefore, we used more stringent cut-off levels resulting in a negative outcome. As most patients in both studies had progressive neurological disease and were tested shortly after start of symptoms and prior to therapy, differences between the study populations do not explain this discrepancy.
Finally, we could not detect HuD-specific circulating CD8+ T cells using Class-I HLA multimers with the same fine specificities as defined by Rousseau et al. [20].
The absence of detectable circulating HuD-specific CD8+ T cells may not be surprising. In a PCR-based study, Plonquet et al. [18] detected the same T-cell clone in neoplastic and nervous tissues, but not in blood. This finding suggests that T cells involved in the pathogenesis of Hu-PNS circulate in concentrations below detection level. An immune response taking place in the central nervous system parenchyma may deplete the circulating pool of CD8+ T cells with that specificity [23]. Furthermore, vaccination studies in melanoma patients demonstrate that clinically effective anti-tumour immune responses may occur despite low levels of melanoma-specific cytotoxic T cells, i.e., below the detection limit of multimer-based assays [4, 16].
In conclusion, we were unable to detect HuD-specific T cells in a large cohort of Hu-PNS patients and controls. However, two of our three assays were designed for the detection of CD8+ T cells only. The IgG1 isotype predominance of serum Hu antibodies in Hu-PNS indicates a T-helper response to the Hu antigen [13]. Therefore, further studies are warranted that focus on the detection of circulating HuD-specific CD4+ T cells. In this context, regulatory CD4+ T cells—which down regulate immune responses towards auto-antigens and tumour-antigens—are of interest. Although the numbers of regulatory T cells are increased in cancer patients [26] and in PNS patients [8] (de Beukelaar et al. unpublished data) their (possibly impaired) function in PNS remains to be studied. Finally, examination of the antigen-specificity of T cells in affected tissues may shed further light on the role of HuD-specific T cells in the pathogenesis of Hu-PNS.
Acknowledgments
This study was supported by a “Revolving Fund” grant from Erasmus MC (Rotterdam, The Netherlands).
References
- 1.Benyahia B, Liblau R, Merle-Beral H, Tourani JM, Dalmau J, Delattre JY. Cell-mediated autoimmunity in paraneoplastic neurological syndromes with anti-Hu antibodies. Ann Neurol. 1999;45:162–167. doi: 10.1002/1531-8249(199902)45:2<162::AID-ANA5>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 2.Bernal F, Graus F, Pifarre A, Saiz A, Benyahia B, Ribalta T. Immunohistochemical analysis of anti-Hu-associated paraneoplastic encephalomyelitis. Acta Neuropathol (Berl) 2002;103:509–515. doi: 10.1007/s00401-001-0498-0. [DOI] [PubMed] [Google Scholar]
- 3.Carpentier AF, Rosenfeld MR, Delattre JY, Whalen RG, Posner JB, Dalmau J. DNA vaccination with HuD inhibits growth of a neuroblastoma in mice. Clin Cancer Res. 1998;4:2819–2824. [PubMed] [Google Scholar]
- 4.Coulie PG, Karanikas V, Colau D, Lurquin C, Landry C, Marchand M, et al. A monoclonal cytolytic T-lymphocyte response observed in a melanoma patient vaccinated with a tumor-specific antigenic peptide encoded by gene MAGE-3. Proc Natl Acad Sci USA. 2001;98:10290–10295. doi: 10.1073/pnas.161260098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Darnell RB. Onconeural antigens and the paraneoplastic neurologic disorders: at the intersection of cancer, immunity, and the brain. Proc Natl Acad Sci USA. 1996;93:4529–4536. doi: 10.1073/pnas.93.10.4529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Darnell RB, Posner JB. Observing the invisible: successful tumor immunity in humans. Nat Immunol. 2003;4:201. doi: 10.1038/ni0303-201. [DOI] [PubMed] [Google Scholar]
- 7.Darnell RB, Posner JB. Paraneoplastic syndromes involving the nervous system. N Engl J Med. 2003;349:1543–1554. doi: 10.1056/NEJMra023009. [DOI] [PubMed] [Google Scholar]
- 8.de Andres C, Esquivel A, de Villoria JG, Graus F, Sanchez-Ramon S. Unusual magnetic resonance imaging and cerebrospinal fluid findings in paraneoplastic cerebellar degeneration: a sequential study. J Neurol Neurosurg Psychiatry. 2006;77:562–563. doi: 10.1136/jnnp.2005.073379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.de Beukelaar JW, Sillevis Smitt PA. Managing paraneoplastic neurological disorders. Oncologist. 2006;11:292–305. doi: 10.1634/theoncologist.11-3-292. [DOI] [PubMed] [Google Scholar]
- 10.de Waal L, Yuksel S, Brandenburg AH, Langedijk JP, Sintnicolaas K, Verjans GM, et al. Identification of a common HLA-DP4-restricted T-cell epitope in the conserved region of the respiratory syncytial virus G protein. J Virol. 2004;78:1775–1781. doi: 10.1128/JVI.78.4.1775-1781.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gratama JW, van Esser JW, Lamers CH, Tournay C, Lowenberg B, Bolhuis RL, et al. Tetramer-based quantification of cytomegalovirus (CMV)-specific CD8+ T lymphocytes in T-cell-depleted stem cell grafts and after transplantation may identify patients at risk for progressive CMV infection. Blood. 2001;98:1358–1364. doi: 10.1182/blood.V98.5.1358. [DOI] [PubMed] [Google Scholar]
- 12.Graus F, Delattre JY, Antoine JC, Dalmau J, Giometto B, Grisold W, et al. Recommended diagnostic criteria for paraneoplastic neurological syndromes. J Neurol Neurosurg Psychiatry. 2004;75:1135–1140. doi: 10.1136/jnnp.2003.034447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jean WC, Dalmau J, Ho A, Posner JB. Analysis of the IgG subclass distribution and inflammatory infiltrates in patients with anti-Hu-associated paraneoplastic encephalomyelitis. Neurology. 1994;44:140–147. doi: 10.1212/wnl.44.1.140. [DOI] [PubMed] [Google Scholar]
- 14.Kern F, Faulhaber N, Frommel C, Khatamzas E, Prosch S, Schonemann C, et al. Analysis of CD8 T cell reactivity to cytomegalovirus using protein-spanning pools of overlapping pentadecapeptides. Eur J Immunol. 2000;30:1676–1682. doi: 10.1002/1521-4141(200006)30:6<1676::AID-IMMU1676>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 15.King PH. Differential expression of the neuroendocrine genes Hel-N1 and HuD in small-cell lung carcinoma: evidence for down-regulation of HuD in the variant phenotype. Int J Cancer. 1997;74:378–382. doi: 10.1002/(SICI)1097-0215(19970822)74:4<378::AID-IJC3>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 16.Knutson KL, dela Rosa C, Disis ML. Laboratory analysis of T-cell immunity. Front Biosci. 2006;11:1932–1944. doi: 10.2741/1936. [DOI] [PubMed] [Google Scholar]
- 17.Manley GT, Smitt PS, Dalmau J, Posner JB. Hu antigens: reactivity with Hu antibodies, tumor expression, and major immunogenic sites. Ann Neurol. 1995;38:102–110. doi: 10.1002/ana.410380117. [DOI] [PubMed] [Google Scholar]
- 18.Plonquet A, Gherardi RK, Creange A, AntoineJC, Benyahia B, Grisold W, et al. Oligoclonal T-cells in blood and target tissues of patients with anti-Hu syndrome. J Neuroimmunol. 2002;122:100–105. doi: 10.1016/S0165-5728(01)00452-0. [DOI] [PubMed] [Google Scholar]
- 19.Plonquet A, Garcia-Pons F, Fernandez E, Philippe C, Marquet J, Rouard H, et al. Peptides derived from the onconeural HuD protein can elicit cytotoxic responses in HHD mouse and human. J Neuroimmunol. 2003;142:93–100. doi: 10.1016/S0165-5728(03)00269-8. [DOI] [PubMed] [Google Scholar]
- 20.Rousseau A, Benyahia B, Dalmau J, Connan F, Guillet JG, Delattre JY, et al. T cell response to Hu-D peptides in patients with anti-Hu syndrome. J Neurooncol. 2005;71:231–236. doi: 10.1007/s11060-004-1723-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sillevis Smitt P, Grefkens J, de Leeuw B, van den Bent M, van Putten W, Hooijkaas H, et al. Survival and outcome in 73 anti-Hu positive patients with paraneoplastic encephalomyelitis/sensory neuronopathy. J Neurol. 2002;249:745–753. doi: 10.1007/s00415-002-0706-4. [DOI] [PubMed] [Google Scholar]
- 22.Sillevis Smitt PA, Manley GT, Posner JB. Immunization with the paraneoplastic encephalomyelitis antigen HuD does not cause neurologic disease in mice. Neurology. 1995;45:1873–1878. doi: 10.1212/wnl.45.10.1873. [DOI] [PubMed] [Google Scholar]
- 23.Stevenson PG, Bangham CR, Hawke S. Recruitment, activation and proliferation of CD8+ memory T cells in an immunoprivileged site. Eur J Immunol. 1997;27:3259–3268. doi: 10.1002/eji.1830271225. [DOI] [PubMed] [Google Scholar]
- 24.Tanaka M, Maruyama Y, Sugie M, Motizuki H, Kamakura K, Tanaka K. Cytotoxic T cell activity against peptides of Hu protein in anti-Hu syndrome. J Neurol Sci. 2002;201:9–12. doi: 10.1016/S0022-510X(02)00157-0. [DOI] [PubMed] [Google Scholar]
- 25.Voltz R, Dalmau J, Posner JB, Rosenfeld MR. T-cell receptor analysis in anti-Hu associated paraneoplastic encephalomyelitis. Neurology. 1998;51:1146–1150. doi: 10.1212/wnl.51.4.1146. [DOI] [PubMed] [Google Scholar]
- 26.Wolf AM, Wolf D, Steurer M, Gastl G, Gunsilius E, Grubeck-Loebenstein B. Increase of regulatory T cells in the peripheral blood of cancer patients. Clin Cancer Res. 2003;9:606–612. [PubMed] [Google Scholar]