Abstract
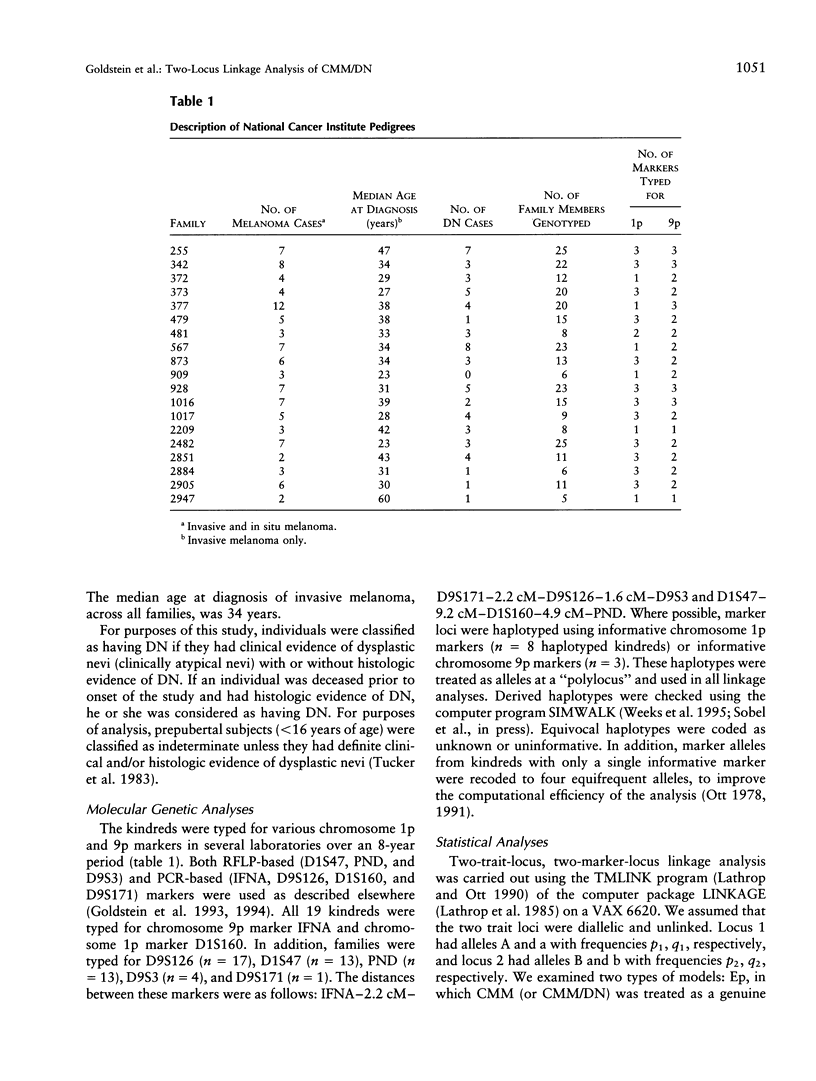
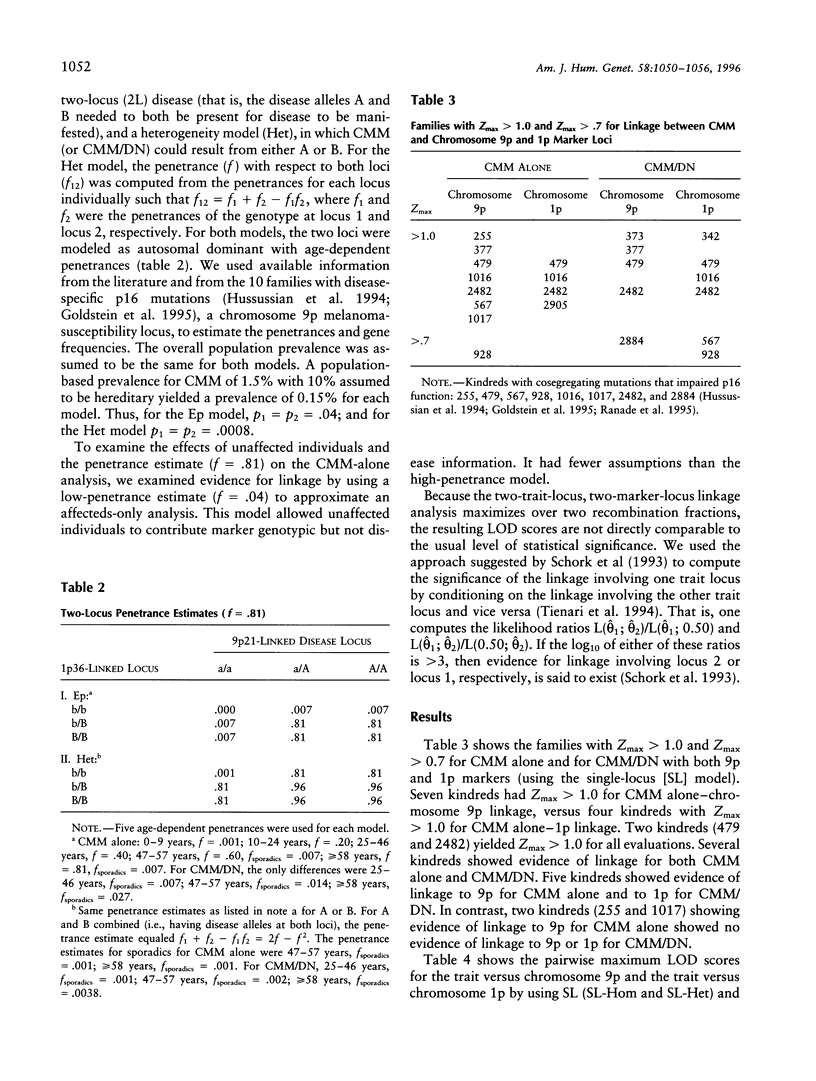
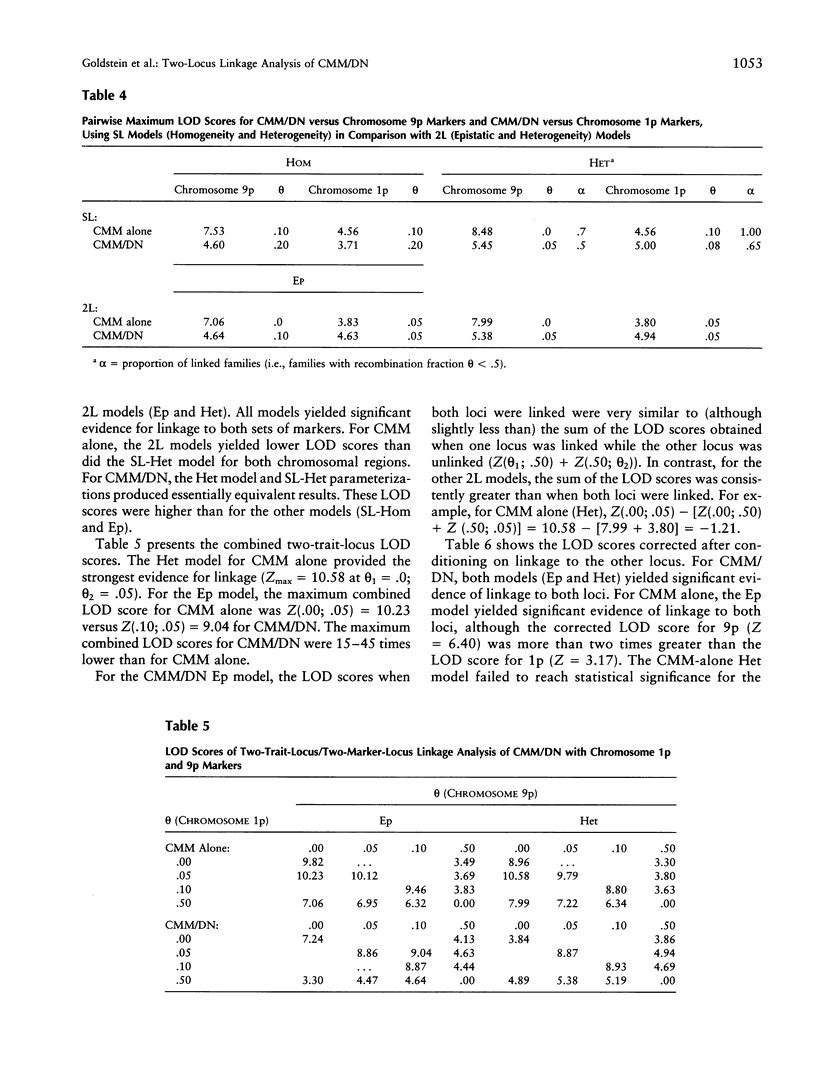
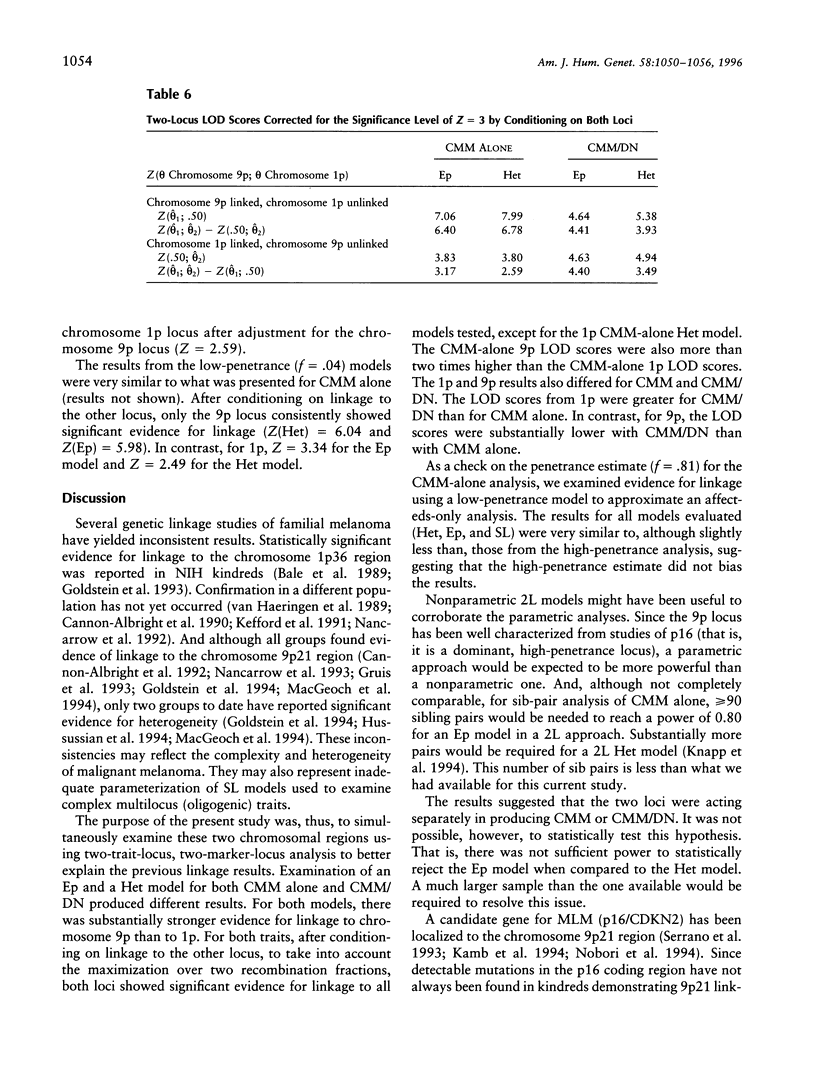
Previous linkage analyses of 19 cutaneous malignant melanoma/dysplastic nevi (CMM/DN) kindreds showed significant evidence of linkage and heterogeneity to both chromosomes 1p and 9p. Five kindreds also showed evidence of linkage (Z>0.7) to both regions. To further examine these findings, we conducted two-trait-locus, two-marker-locus linkage analysis. We examined one homogeneity and one heterogeneity single-locus model (SL-Hom and SL-Het), and two-locus (2L) models: an epistatic model (Ep), in which CMM was treated as a genuine 2L disease, and a heterogeneity model (Het), in which CMM could result from disease alleles at either locus. Both loci were modeled as autosomal dominant. The LOD scores for CMM alone were highest using the SL-Het model (Z = 8.48, theta = .0). There was much stronger evidence of linkage to chromosome 9p than to 1p for CMM alone; the LOD scores were approximately two times greater on 9p than on 1p. The change in LOD scores from an evaluation of CMM alone to CMM/DN suggested that a chromosome 1p locus (or loci) contributed to both CMM and CMM/DN, whereas a 9p locus contributed more to CMM alone. For both 2L models, the LOD scores from 1p were greater for CMM/DN than for CMM alone (Ep: Z=4.63 vs. 3.83; Het: 4.94 vs. 3.80, respectively). In contrast, for 9p, the LOD scores were substantially lower with CMM/DN than with CMM alone (Ep: 4.64 vs. 7.06; Het: 5.38 vs. 7.99, respectively). After conditioning on linkage to the other locus, only the 9p locus consistently showed significant evidence for linkage to CMM alone. Thus, the application of 2L models may be useful to help unravel the complexities of familial melanoma.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bale S. J., Dracopoli N. C., Tucker M. A., Clark W. H., Jr, Fraser M. C., Stanger B. Z., Green P., Donis-Keller H., Housman D. E., Greene M. H. Mapping the gene for hereditary cutaneous malignant melanoma-dysplastic nevus to chromosome 1p. N Engl J Med. 1989 May 25;320(21):1367–1372. doi: 10.1056/NEJM198905253202102. [DOI] [PubMed] [Google Scholar]
- Cannon-Albright L. A., Goldgar D. E., Meyer L. J., Lewis C. M., Anderson D. E., Fountain J. W., Hegi M. E., Wiseman R. W., Petty E. M., Bale A. E. Assignment of a locus for familial melanoma, MLM, to chromosome 9p13-p22. Science. 1992 Nov 13;258(5085):1148–1152. doi: 10.1126/science.1439824. [DOI] [PubMed] [Google Scholar]
- Cannon-Albright L. A., Goldgar D. E., Wright E. C., Turco A., Jost M., Meyer L. J., Piepkorn M., Zone J. J., Skolnick M. H. Evidence against the reported linkage of the cutaneous melanoma-dysplastic nevus syndrome locus to chromosome Ip36. Am J Hum Genet. 1990 May;46(5):912–918. [PMC free article] [PubMed] [Google Scholar]
- Cannon-Albright L. A., Meyer L. J., Goldgar D. E., Lewis C. M., McWhorter W. P., Jost M., Harrison D., Anderson D. E., Zone J. J., Skolnick M. H. Penetrance and expressivity of the chromosome 9p melanoma susceptibility locus (MLM). Cancer Res. 1994 Dec 1;54(23):6041–6044. [PubMed] [Google Scholar]
- Goldstein A. M., Dracopoli N. C., Engelstein M., Fraser M. C., Clark W. H., Jr, Tucker M. A. Linkage of cutaneous malignant melanoma/dysplastic nevi to chromosome 9p, and evidence for genetic heterogeneity. Am J Hum Genet. 1994 Mar;54(3):489–496. [PMC free article] [PubMed] [Google Scholar]
- Goldstein A. M., Dracopoli N. C., Ho E. C., Fraser M. C., Kearns K. S., Bale S. J., McBride O. W., Clark W. H., Jr, Tucker M. A. Further evidence for a locus for cutaneous malignant melanoma-dysplastic nevus (CMM/DN) on chromosome 1p, and evidence for genetic heterogeneity. Am J Hum Genet. 1993 Mar;52(3):537–550. [PMC free article] [PubMed] [Google Scholar]
- Goldstein A. M., Fraser M. C., Struewing J. P., Hussussian C. J., Ranade K., Zametkin D. P., Fontaine L. S., Organic S. M., Dracopoli N. C., Clark W. H., Jr Increased risk of pancreatic cancer in melanoma-prone kindreds with p16INK4 mutations. N Engl J Med. 1995 Oct 12;333(15):970–974. doi: 10.1056/NEJM199510123331504. [DOI] [PubMed] [Google Scholar]
- Hussussian C. J., Struewing J. P., Goldstein A. M., Higgins P. A., Ally D. S., Sheahan M. D., Clark W. H., Jr, Tucker M. A., Dracopoli N. C. Germline p16 mutations in familial melanoma. Nat Genet. 1994 Sep;8(1):15–21. doi: 10.1038/ng0994-15. [DOI] [PubMed] [Google Scholar]
- Kamb A., Gruis N. A., Weaver-Feldhaus J., Liu Q., Harshman K., Tavtigian S. V., Stockert E., Day R. S., 3rd, Johnson B. E., Skolnick M. H. A cell cycle regulator potentially involved in genesis of many tumor types. Science. 1994 Apr 15;264(5157):436–440. doi: 10.1126/science.8153634. [DOI] [PubMed] [Google Scholar]
- Kefford R. F., Salmon J., Shaw H. M., Donald J. A., McCarthy W. H. Hereditary melanoma in Australia. Variable association with dysplastic nevi and absence of genetic linkage to chromosome 1p. Cancer Genet Cytogenet. 1991 Jan;51(1):45–55. doi: 10.1016/0165-4608(91)90007-h. [DOI] [PubMed] [Google Scholar]
- Knapp M., Seuchter S. A., Baur M. P. Two-locus disease models with two marker loci: the power of affected-sib-pair tests. Am J Hum Genet. 1994 Nov;55(5):1030–1041. [PMC free article] [PubMed] [Google Scholar]
- Lathrop G. M., Lalouel J. M., Julier C., Ott J. Multilocus linkage analysis in humans: detection of linkage and estimation of recombination. Am J Hum Genet. 1985 May;37(3):482–498. [PMC free article] [PubMed] [Google Scholar]
- MacGeoch C., Bishop J. A., Bataille V., Bishop D. T., Frischauf A. M., Meloni R., Cuzick J., Pinney E., Spurr N. K. Genetic heterogeneity in familial malignant melanoma. Hum Mol Genet. 1994 Dec;3(12):2195–2200. doi: 10.1093/hmg/3.12.2195. [DOI] [PubMed] [Google Scholar]
- Nancarrow D. J., Mann G. J., Holland E. A., Walker G. J., Beaton S. C., Walters M. K., Luxford C., Palmer J. M., Donald J. A., Weber J. L. Confirmation of chromosome 9p linkage in familial melanoma. Am J Hum Genet. 1993 Oct;53(4):936–942. [PMC free article] [PubMed] [Google Scholar]
- Nancarrow D. J., Palmer J. M., Walters M. K., Kerr B. M., Hafner G. J., Garske L., McLeod G. R., Hayward N. K. Exclusion of the familial melanoma locus (MLM) from the PND/D1S47 and MYCL1 regions of chromosome arm 1p in 7 Australian pedigrees. Genomics. 1992 Jan;12(1):18–25. doi: 10.1016/0888-7543(92)90401-d. [DOI] [PubMed] [Google Scholar]
- Nobori T., Miura K., Wu D. J., Lois A., Takabayashi K., Carson D. A. Deletions of the cyclin-dependent kinase-4 inhibitor gene in multiple human cancers. Nature. 1994 Apr 21;368(6473):753–756. doi: 10.1038/368753a0. [DOI] [PubMed] [Google Scholar]
- Ott J. A simple scheme for the analysis of HLA linkages in pedigrees. Ann Hum Genet. 1978 Oct;42(2):255–257. doi: 10.1111/j.1469-1809.1978.tb00657.x. [DOI] [PubMed] [Google Scholar]
- Ranade K., Hussussian C. J., Sikorski R. S., Varmus H. E., Goldstein A. M., Tucker M. A., Serrano M., Hannon G. J., Beach D., Dracopoli N. C. Mutations associated with familial melanoma impair p16INK4 function. Nat Genet. 1995 May;10(1):114–116. doi: 10.1038/ng0595-114. [DOI] [PubMed] [Google Scholar]
- Schork N. J., Boehnke M., Terwilliger J. D., Ott J. Two-trait-locus linkage analysis: a powerful strategy for mapping complex genetic traits. Am J Hum Genet. 1993 Nov;53(5):1127–1136. [PMC free article] [PubMed] [Google Scholar]
- Serrano M., Gómez-Lahoz E., DePinho R. A., Beach D., Bar-Sagi D. Inhibition of ras-induced proliferation and cellular transformation by p16INK4. Science. 1995 Jan 13;267(5195):249–252. doi: 10.1126/science.7809631. [DOI] [PubMed] [Google Scholar]
- Serrano M., Hannon G. J., Beach D. A new regulatory motif in cell-cycle control causing specific inhibition of cyclin D/CDK4. Nature. 1993 Dec 16;366(6456):704–707. doi: 10.1038/366704a0. [DOI] [PubMed] [Google Scholar]
- Sherr C. J. Mammalian G1 cyclins. Cell. 1993 Jun 18;73(6):1059–1065. doi: 10.1016/0092-8674(93)90636-5. [DOI] [PubMed] [Google Scholar]
- Tienari P. J., Terwilliger J. D., Ott J., Palo J., Peltonen L. Two-locus linkage analysis in multiple sclerosis (MS). Genomics. 1994 Jan 15;19(2):320–325. doi: 10.1006/geno.1994.1064. [DOI] [PubMed] [Google Scholar]
- Tucker M. A., Greene M. H., Clark W. H., Jr, Kraemer K. H., Fraser M. C., Elder D. E. Dysplastic nevi on the scalp of prepubertal children from melanoma-prone families. J Pediatr. 1983 Jul;103(1):65–69. doi: 10.1016/s0022-3476(83)80777-x. [DOI] [PubMed] [Google Scholar]
- Walker G. J., Hussussian C. J., Flores J. F., Glendening J. M., Haluska F. G., Dracopoli N. C., Hayward N. K., Fountain J. W. Mutations of the CDKN2/p16INK4 gene in Australian melanoma kindreds. Hum Mol Genet. 1995 Oct;4(10):1845–1852. doi: 10.1093/hmg/4.10.1845. [DOI] [PubMed] [Google Scholar]
- Weeks D. E., Sobel E., O'Connell J. R., Lange K. Computer programs for multilocus haplotyping of general pedigrees. Am J Hum Genet. 1995 Jun;56(6):1506–1507. [PMC free article] [PubMed] [Google Scholar]
- Wölfel T., Hauer M., Schneider J., Serrano M., Wölfel C., Klehmann-Hieb E., De Plaen E., Hankeln T., Meyer zum Büschenfelde K. H., Beach D. A p16INK4a-insensitive CDK4 mutant targeted by cytolytic T lymphocytes in a human melanoma. Science. 1995 Sep 1;269(5228):1281–1284. doi: 10.1126/science.7652577. [DOI] [PubMed] [Google Scholar]
- Zuo L., Weger J., Yang Q., Goldstein A. M., Tucker M. A., Walker G. J., Hayward N., Dracopoli N. C. Germline mutations in the p16INK4a binding domain of CDK4 in familial melanoma. Nat Genet. 1996 Jan;12(1):97–99. doi: 10.1038/ng0196-97. [DOI] [PubMed] [Google Scholar]
- van Haeringen A., Bergman W., Nelen M. R., van der Kooij-Meijs E., Hendrikse I., Wijnen J. T., Khan P. M., Klasen E. C., Frants R. R. Exclusion of the dysplastic nevus syndrome (DNS) locus from the short arm of chromosome 1 by linkage studies in Dutch families. Genomics. 1989 Jul;5(1):61–64. doi: 10.1016/0888-7543(89)90086-4. [DOI] [PubMed] [Google Scholar]


