Abstract
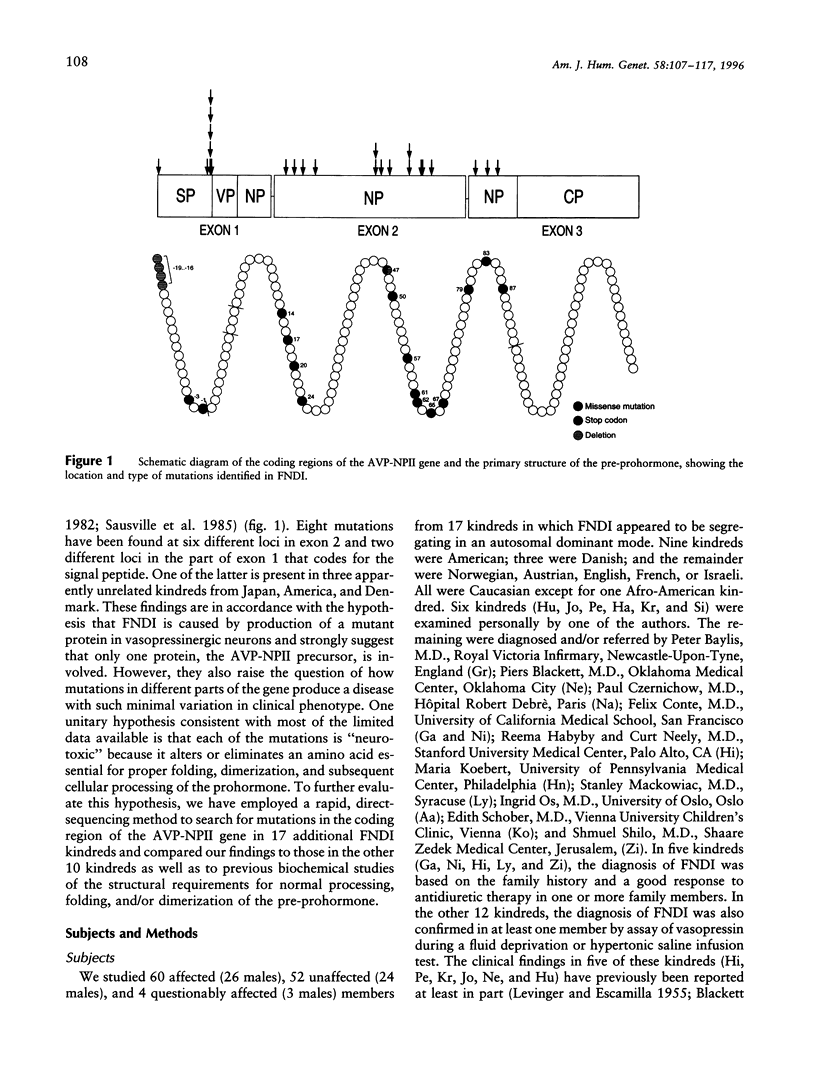
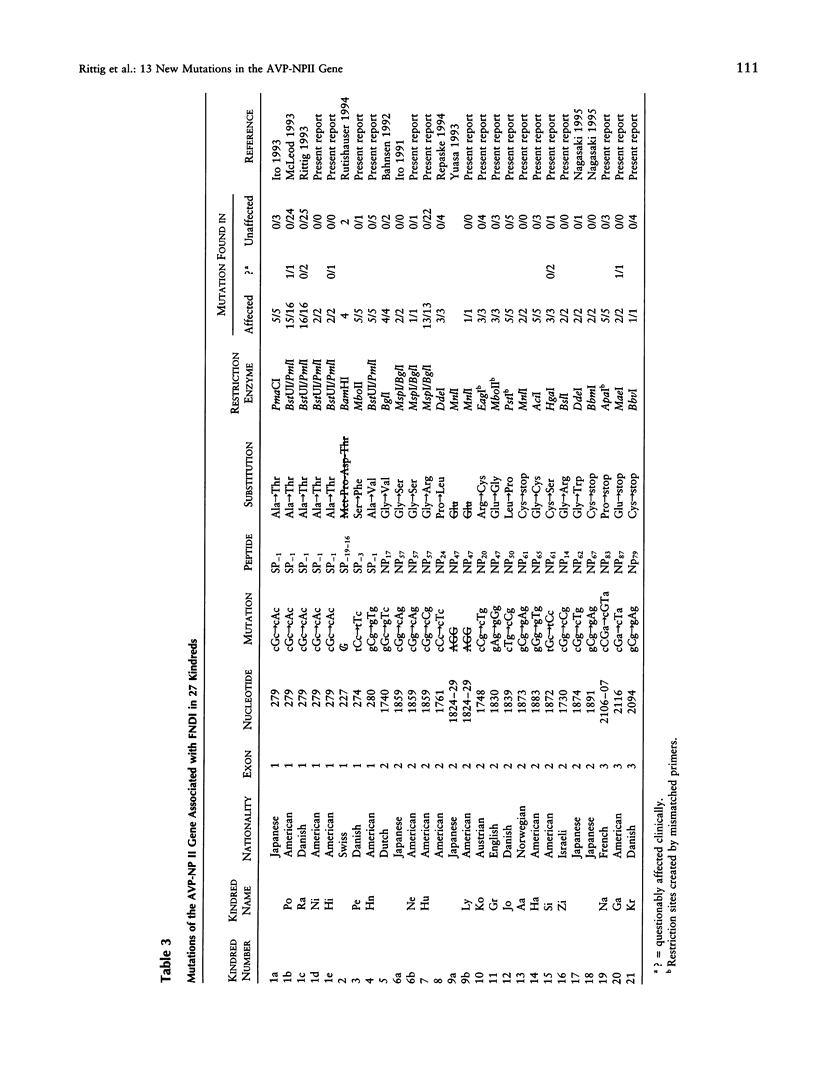
Familial neurohypophyseal diabetes insipidus (FNDI) is an autosomal dominant disorder characterized by progressive postnatal deficiency of arginine vasopressin as a result of mutation in the gene that encodes the hormone. To determine the extent of mutations in the coding region that produce the phenotype, we studied members of 17 unrelated kindreds with the disorder. We sequenced all 3 exons of the gene by using a rapid, direct dye-terminator method and found the causative mutation in each kindred. In four kindreds, the mutations were each identical to mutations described in other affected families. In the other 13 kindreds each mutation was unique. There were two missense mutations that altered the cleavage region of the signal peptide, seven missense mutations in exon 2, which codes for the conserved portion of the protein, one nonsense mutation in exon 2, and three nonsense mutations in exon 3. These findings, together with the clinical features of FNDI, suggest that each of the mutations exerts an effect by directing the production of a pre-prohormone that cannot be folded, processed, or degraded properly and eventually destroys vasopressinergic neurons.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BRAVERMAN L. E., MANCINI J. P., MCGOLDRICK D. M. HEREDITARY IDIOPATHIC DIABETES INSIPIDUS. A CASE REPORT WITH AUTOPSY FINDINGS. Ann Intern Med. 1965 Sep;63:503–508. doi: 10.7326/0003-4819-63-3-503. [DOI] [PubMed] [Google Scholar]
- Bahnsen U., Oosting P., Swaab D. F., Nahke P., Richter D., Schmale H. A missense mutation in the vasopressin-neurophysin precursor gene cosegregates with human autosomal dominant neurohypophyseal diabetes insipidus. EMBO J. 1992 Jan;11(1):19–23. doi: 10.1002/j.1460-2075.1992.tb05022.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylis P. H., Robertson G. L. Vasopressin function in familial cranial diabetes insipidus. Postgrad Med J. 1981 Jan;57(663):36–40. doi: 10.1136/pgmj.57.663.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker J., Craig E. A. Heat-shock proteins as molecular chaperones. Eur J Biochem. 1994 Jan 15;219(1-2):11–23. doi: 10.1007/978-3-642-79502-2_2. [DOI] [PubMed] [Google Scholar]
- Blackett P. R., Seif S. M., Altmiller D. H., Robinson A. G. Familial central diabetes insipidus: vasopressin and nicotine stimulated neurophysin deficiency with subnormal oxytocin and estrogen stimulated neurophysin. Am J Med Sci. 1983 Nov-Dec;286(3):42–46. doi: 10.1097/00000441-198311000-00007. [DOI] [PubMed] [Google Scholar]
- Block L. H., Furrer J., Locher R. A., Siegenthaler W., Vetter W. Changes in tissue sensitivity to vasopressin in hereditary hypothalamic diabetes insipidus. Klin Wochenschr. 1981 Aug 3;59(15):831–836. doi: 10.1007/BF01721052. [DOI] [PubMed] [Google Scholar]
- Böhni P. C., Deshaies R. J., Schekman R. W. SEC11 is required for signal peptide processing and yeast cell growth. J Cell Biol. 1988 Apr;106(4):1035–1042. doi: 10.1083/jcb.106.4.1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L. Q., Rose J. P., Breslow E., Yang D., Chang W. R., Furey W. F., Jr, Sax M., Wang B. C. Crystal structure of a bovine neurophysin II dipeptide complex at 2.8 A determined from the single-wavelength anomalous scattering signal of an incorporated iodine atom. Proc Natl Acad Sci U S A. 1991 May 15;88(10):4240–4244. doi: 10.1073/pnas.88.10.4240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cyr D. M., Langer T., Douglas M. G. DnaJ-like proteins: molecular chaperones and specific regulators of Hsp70. Trends Biochem Sci. 1994 Apr;19(4):176–181. doi: 10.1016/0968-0004(94)90281-x. [DOI] [PubMed] [Google Scholar]
- Dalbey R. E., Von Heijne G. Signal peptidases in prokaryotes and eukaryotes--a new protease family. Trends Biochem Sci. 1992 Nov;17(11):474–478. doi: 10.1016/0968-0004(92)90492-r. [DOI] [PubMed] [Google Scholar]
- Driedger A. A., Linton A. L. Familial ADH-responsive diabetes insipidus: response to thiazides and chlorpropamide. Can Med Assoc J. 1973 Oct 6;109(7):594–597. [PMC free article] [PubMed] [Google Scholar]
- FRASER F. C. Hérédité dominante d'un diabète insipide dû à une déficience en pitressine. J Genet Hum. 1955 Dec;4(4):195–203. [PubMed] [Google Scholar]
- Fikes J. D., Barkocy-Gallagher G. A., Klapper D. G., Bassford P. J., Jr Maturation of Escherichia coli maltose-binding protein by signal peptidase I in vivo. Sequence requirements for efficient processing and demonstration of an alternate cleavage site. J Biol Chem. 1990 Feb 25;265(6):3417–3423. [PubMed] [Google Scholar]
- Folz R. J., Nothwehr S. F., Gordon J. I. Substrate specificity of eukaryotic signal peptidase. Site-saturation mutagenesis at position -1 regulates cleavage between multiple sites in human pre (delta pro) apolipoprotein A-II. J Biol Chem. 1988 Feb 5;263(4):2070–2078. [PubMed] [Google Scholar]
- Gaut J. R., Hendershot L. M. The modification and assembly of proteins in the endoplasmic reticulum. Curr Opin Cell Biol. 1993 Aug;5(4):589–595. doi: 10.1016/0955-0674(93)90127-c. [DOI] [PubMed] [Google Scholar]
- Green J. R., Buchan G. C., Alvord E. C., Jr, Swanson A. G. Heredtary and idiopathic types of diabetes insipidus. Brain. 1967 Sep;90(3):707–714. doi: 10.1093/brain/90.3.707. [DOI] [PubMed] [Google Scholar]
- Gregersen N., Blakemore A. I., Winter V., Andresen B., Kølvraa S., Bolund L., Curtis D., Engel P. C. Specific diagnosis of medium-chain acyl-CoA dehydrogenase (MCAD) deficiency in dried blood spots by a polymerase chain reaction (PCR) assay detecting a point-mutation (G985) in the MCAD gene. Clin Chim Acta. 1991 Nov 9;203(1):23–34. doi: 10.1016/0009-8981(91)90153-4. [DOI] [PubMed] [Google Scholar]
- Halban P. A., Irminger J. C. Sorting and processing of secretory proteins. Biochem J. 1994 Apr 1;299(Pt 1):1–18. doi: 10.1042/bj2990001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helenius A., Marquardt T., Braakman I. The endoplasmic reticulum as a protein-folding compartment. Trends Cell Biol. 1992 Aug;2(8):227–231. doi: 10.1016/0962-8924(92)90309-b. [DOI] [PubMed] [Google Scholar]
- Hortin G., Boime I. Miscleavage at the presequence of rat preprolactin synthesized in pituitary cells incubated with a threonine analog. Cell. 1981 May;24(2):453–461. doi: 10.1016/0092-8674(81)90336-6. [DOI] [PubMed] [Google Scholar]
- Ito M., Mori Y., Oiso Y., Saito H. A single base substitution in the coding region for neurophysin II associated with familial central diabetes insipidus. J Clin Invest. 1991 Feb;87(2):725–728. doi: 10.1172/JCI115052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito M., Oiso Y., Murase T., Kondo K., Saito H., Chinzei T., Racchi M., Lively M. O. Possible involvement of inefficient cleavage of preprovasopressin by signal peptidase as a cause for familial central diabetes insipidus. J Clin Invest. 1993 Jun;91(6):2565–2571. doi: 10.1172/JCI116494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen H. K., Jensen T. G., Jensen L. G., Hansen P. S., Kjeldsen M., Andresen B. S., Nielsen V., Meinertz H., Hansen A. B., Bolund L. Characterization of a disease-causing Glu119-Lys mutation in the low-density lipoprotein receptor gene in two Danish families with heterozygous familial hypercholesterolemia. Hum Mutat. 1994;4(2):102–113. doi: 10.1002/humu.1380040203. [DOI] [PubMed] [Google Scholar]
- Kaplowitz P. B., D'Ercole A. J., Robertson G. L. Radioimmunoassay of vasopressin in familial cental diabetes insipidus. J Pediatr. 1982 Jan;100(1):76–81. doi: 10.1016/s0022-3476(82)80238-2. [DOI] [PubMed] [Google Scholar]
- LEVINGER E. L., ESCAMILLA R. F. Hereditary diabetes insipidus: report of 20 cases in seven generations. J Clin Endocrinol Metab. 1955 May;15(5):547–552. doi: 10.1210/jcem-15-5-547. [DOI] [PubMed] [Google Scholar]
- Laforet G. A., Kendall D. A. Functional limits of conformation, hydrophobicity, and steric constraints in prokaryotic signal peptide cleavage regions. Wild type transport by a simple polymeric signal sequence. J Biol Chem. 1991 Jan 15;266(2):1326–1334. [PubMed] [Google Scholar]
- Land H., Schütz G., Schmale H., Richter D. Nucleotide sequence of cloned cDNA encoding bovine arginine vasopressin-neurophysin II precursor. Nature. 1982 Jan 28;295(5847):299–303. doi: 10.1038/295299a0. [DOI] [PubMed] [Google Scholar]
- MARTIN F. I. Familial diabetes insipidus. Q J Med. 1959 Oct;28:573–582. [PubMed] [Google Scholar]
- Marquardt T., Helenius A. Misfolding and aggregation of newly synthesized proteins in the endoplasmic reticulum. J Cell Biol. 1992 May;117(3):505–513. doi: 10.1083/jcb.117.3.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLeod J. F., Kovács L., Gaskill M. B., Rittig S., Bradley G. S., Robertson G. L. Familial neurohypophyseal diabetes insipidus associated with a signal peptide mutation. J Clin Endocrinol Metab. 1993 Sep;77(3):599A–599G. doi: 10.1210/jcem.77.3.8370682. [DOI] [PubMed] [Google Scholar]
- Meinders A. E., Bijlsma J. B. A family with congenital hypothalamic neurohypophyseal diabetes insipidus. Folia Med Neerl. 1970;13(2):68–72. [PubMed] [Google Scholar]
- Mohr E., Hillers M., Ivell R., Haulica I. D., Richter D. Expression of the vasopressin and oxytocin genes in human hypothalami. FEBS Lett. 1985 Nov 25;193(1):12–16. doi: 10.1016/0014-5793(85)80069-7. [DOI] [PubMed] [Google Scholar]
- Müller M. Proteolysis in protein import and export: signal peptide processing in eu- and prokaryotes. Experientia. 1992 Feb 15;48(2):118–129. doi: 10.1007/BF01923506. [DOI] [PubMed] [Google Scholar]
- Nagahora H., Fujisawa H., Jigami Y. Alterations in the cleavage site of the signal sequence for the secretion of human lysozyme by Saccharomyces cerevisiae. FEBS Lett. 1988 Oct 10;238(2):329–332. doi: 10.1016/0014-5793(88)80506-4. [DOI] [PubMed] [Google Scholar]
- Nagasaki H., Ito M., Yuasa H., Saito H., Fukase M., Hamada K., Ishikawa E., Katakami H., Oiso Y. Two novel mutations in the coding region for neurophysin-II associated with familial central diabetes insipidus. J Clin Endocrinol Metab. 1995 Apr;80(4):1352–1356. doi: 10.1210/jcem.80.4.7714110. [DOI] [PubMed] [Google Scholar]
- Nothwehr S. F., Gordon J. I. Structural features in the NH2-terminal region of a model eukaryotic signal peptide influence the site of its cleavage by signal peptidase. J Biol Chem. 1990 Oct 5;265(28):17202–17208. [PubMed] [Google Scholar]
- Os I., Aakesson I., Enger E. Plasma vasopressin in hereditary cranial diabetes insipidus. Acta Med Scand. 1985;217(4):429–434. doi: 10.1111/j.0954-6820.1985.tb02719.x. [DOI] [PubMed] [Google Scholar]
- PENDER C. B., FRASER F. C. Dominant inheritance of diabetes insipidus; a family study. Pediatrics. 1953 Mar;11(3):246–254. [PubMed] [Google Scholar]
- Pedersen E. B., Danielsen H., Spencer E. S. Effect of indapamide on renal plasma flow, glomerular filtration rate and arginine vasopressin in plasma in essential hypertension. Eur J Clin Pharmacol. 1984;26(5):543–547. doi: 10.1007/BF00543482. [DOI] [PubMed] [Google Scholar]
- Pedersen E. B., Lamm L. U., Albertsen K., Madsen M., Bruun-Petersen G., Henningsen K., Friedrich U., Magnusson K. Familial cranial diabetes insipidus: a report of five families. Genetic, diagnostic and therapeutic aspects. Q J Med. 1985 Dec;57(224):883–896. [PubMed] [Google Scholar]
- Prabhakaran M. The distribution of physical, chemical and conformational properties in signal and nascent peptides. Biochem J. 1990 Aug 1;269(3):691–696. doi: 10.1042/bj2690691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Repaske D. R., Browning J. E. A de novo mutation in the coding sequence for neurophysin-II (Pro24-->Leu) is associated with onset and transmission of autosomal dominant neurohypophyseal diabetes insipidus. J Clin Endocrinol Metab. 1994 Aug;79(2):421–427. doi: 10.1210/jcem.79.2.8045958. [DOI] [PubMed] [Google Scholar]
- Riddell D. C., Mallonee R., Phillips J. A., Parks J. S., Sexton L. A., Hamerton J. L. Chromosomal assignment of human sequences encoding arginine vasopressin-neurophysin II and growth hormone releasing factor. Somat Cell Mol Genet. 1985 Mar;11(2):189–195. doi: 10.1007/BF01534707. [DOI] [PubMed] [Google Scholar]
- Robertson G. L., Mahr E. A., Athar S., Sinha T. Development and clinical application of a new method for the radioimmunoassay of arginine vasopressin in human plasma. J Clin Invest. 1973 Sep;52(9):2340–2352. doi: 10.1172/JCI107423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson G. L. The use of vasopressin assays in physiology and pathophysiology. Semin Nephrol. 1994 Jul;14(4):368–383. [PubMed] [Google Scholar]
- Rutherford S. L., Zuker C. S. Protein folding and the regulation of signaling pathways. Cell. 1994 Dec 30;79(7):1129–1132. doi: 10.1016/0092-8674(94)90003-5. [DOI] [PubMed] [Google Scholar]
- Sausville E., Carney D., Battey J. The human vasopressin gene is linked to the oxytocin gene and is selectively expressed in a cultured lung cancer cell line. J Biol Chem. 1985 Aug 25;260(18):10236–10241. [PubMed] [Google Scholar]
- Shelness G. S., Lin L., Nicchitta C. V. Membrane topology and biogenesis of eukaryotic signal peptidase. J Biol Chem. 1993 Mar 5;268(7):5201–5208. [PubMed] [Google Scholar]
- Toth E. L., Bowen P. A., Crockford P. M. Hereditary central diabetes insipidus: plasma levels of antidiuretic hormone in a family with a possible osmoreceptor defect. Can Med Assoc J. 1984 Nov 15;131(10):1237–1241. [PMC free article] [PubMed] [Google Scholar]
- Urade R., Kito M. Inhibition by acidic phospholipids of protein degradation by ER-60 protease, a novel cysteine protease, of endoplasmic reticulum. FEBS Lett. 1992 Nov 2;312(1):83–86. doi: 10.1016/0014-5793(92)81415-i. [DOI] [PubMed] [Google Scholar]
- Wu Y., Whitman I., Molmenti E., Moore K., Hippenmeyer P., Perlmutter D. H. A lag in intracellular degradation of mutant alpha 1-antitrypsin correlates with the liver disease phenotype in homozygous PiZZ alpha 1-antitrypsin deficiency. Proc Natl Acad Sci U S A. 1994 Sep 13;91(19):9014–9018. doi: 10.1073/pnas.91.19.9014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuasa H., Ito M., Nagasaki H., Oiso Y., Miyamoto S., Sasaki N., Saito H. Glu-47, which forms a salt bridge between neurophysin-II and arginine vasopressin, is deleted in patients with familial central diabetes insipidus. J Clin Endocrinol Metab. 1993 Sep;77(3):600–604. doi: 10.1210/jcem.77.3.8103767. [DOI] [PubMed] [Google Scholar]
- von Heijne G. How signal sequences maintain cleavage specificity. J Mol Biol. 1984 Feb 25;173(2):243–251. doi: 10.1016/0022-2836(84)90192-x. [DOI] [PubMed] [Google Scholar]