Abstract
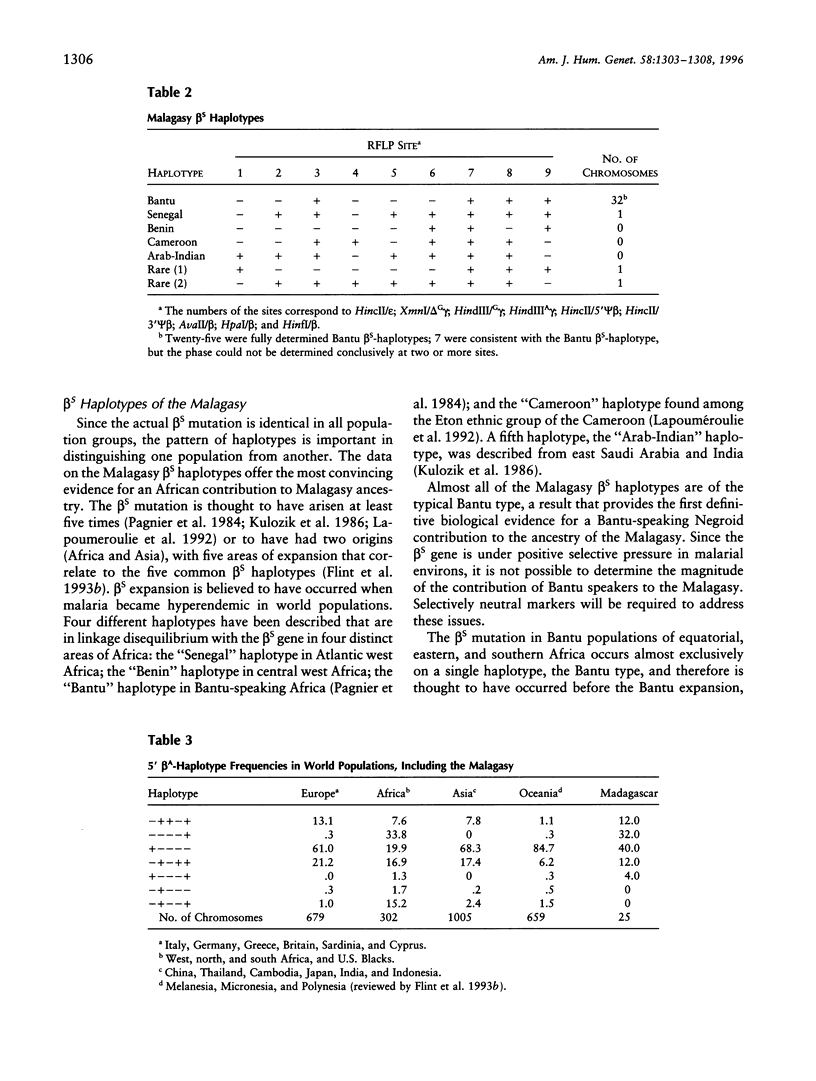
The origins of the inhabitants of Madagascar have not been fully resolved. Anthropological studies and preliminary genetic data point to two main sources of ancestry of the Malagasy, namely, Indonesian and African, with additional contributions from India and Arabia. The sickle-cell (beta s) mutation is found in populations of African and Indian origin. The frequency of the beta s-globin gene, derived from 1,425 Malagasy individuals, varies from 0 in some highland populations to .25 in some coastal populations. The beta s mutation is thought to have arisen at least five times, on the basis of the presence of five distinct beta s-associated haplotypes, each found in a separate geographic area. Twenty-five of the 35 Malagasy beta s haplotypes were of the typical "Bantu" type, 1 "Senegal" haplotype was found, and 2 rare or atypical haplotypes were observed; the remaining 7 haplotypes were consistent with the Bantu haplotype. The Bantu beta s mutation is thought to have been introduced into Madagascar by Bantu-speaking immigrants (colonists or slaves) from central or east Africa. The Senegal beta s mutation may have been introduced to the island via Portuguese naval explorers. This study provides the first definitive biological evidence that a major component of Malagasy ancestry is derived from African populations, in particular, Bantu-speaking Negroids. beta A haplotypes are also consistent with the claim for a significant African contribution to Malagasy ancestry but are also suggestive of Asian/Oceanic and Caucasoid admixture within the Malagasy population.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Burns A. L., Spence S., Kosche K., Ramirez F., Mears J. G., Schreiner H., Miller C., Baird M., Leibowitz D., Giardina P. Isolation and characterization of cloned DNA: the delta and beta globin genes in homozygous beta + thalassemia. Blood. 1981 Jan;57(1):140–146. [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Flint J., Harding R. M., Boyce A. J., Clegg J. B. The population genetics of the haemoglobinopathies. Baillieres Clin Haematol. 1993 Mar;6(1):215–262. doi: 10.1016/s0950-3536(05)80071-x. [DOI] [PubMed] [Google Scholar]
- Flint J., Harding R. M., Clegg J. B., Boyce A. J. Why are some genetic diseases common? Distinguishing selection from other processes by molecular analysis of globin gene variants. Hum Genet. 1993 Mar;91(2):91–117. doi: 10.1007/BF00222709. [DOI] [PubMed] [Google Scholar]
- Gilman J. G., Huisman T. H. DNA sequence variation associated with elevated fetal G gamma globin production. Blood. 1985 Oct;66(4):783–787. [PubMed] [Google Scholar]
- Kulozik A. E., Wainscoat J. S., Serjeant G. R., Kar B. C., Al-Awamy B., Essan G. J., Falusi A. G., Haque S. K., Hilali A. M., Kate S. Geographical survey of beta S-globin gene haplotypes: evidence for an independent Asian origin of the sickle-cell mutation. Am J Hum Genet. 1986 Aug;39(2):239–244. [PMC free article] [PubMed] [Google Scholar]
- Lapouméroulie C., Dunda O., Ducrocq R., Trabuchet G., Mony-Lobé M., Bodo J. M., Carnevale P., Labie D., Elion J., Krishnamoorthy R. A novel sickle cell mutation of yet another origin in Africa: the Cameroon type. Hum Genet. 1992 May;89(3):333–337. doi: 10.1007/BF00220553. [DOI] [PubMed] [Google Scholar]
- Miller S. A., Dykes D. D., Polesky H. F. A simple salting out procedure for extracting DNA from human nucleated cells. Nucleic Acids Res. 1988 Feb 11;16(3):1215–1215. doi: 10.1093/nar/16.3.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monteiro C., Rueff J., Falcao A. B., Portugal S., Weatherall D. J., Kulozik A. E. The frequency and origin of the sickle cell mutation in the district of Coruche/Portugal. Hum Genet. 1989 Jun;82(3):255–258. doi: 10.1007/BF00291165. [DOI] [PubMed] [Google Scholar]
- Nagel R. L., Fleming A. F. Genetic epidemiology of the beta s gene. Baillieres Clin Haematol. 1992 Apr;5(2):331–365. doi: 10.1016/s0950-3536(11)80023-5. [DOI] [PubMed] [Google Scholar]
- Nagel R. L., Ranney H. M. Genetic epidemiology of structural mutations of the beta-globin gene. Semin Hematol. 1990 Oct;27(4):342–359. [PubMed] [Google Scholar]
- Old J. M., Ludlam C. A. Antenatal diagnosis. Baillieres Clin Haematol. 1991 Apr;4(2):391–428. doi: 10.1016/s0950-3536(05)80165-9. [DOI] [PubMed] [Google Scholar]
- Pagnier J., Mears J. G., Dunda-Belkhodja O., Schaefer-Rego K. E., Beldjord C., Nagel R. L., Labie D. Evidence for the multicentric origin of the sickle cell hemoglobin gene in Africa. Proc Natl Acad Sci U S A. 1984 Mar;81(6):1771–1773. doi: 10.1073/pnas.81.6.1771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SINGER R., BUDTZ-OLSEN O. E., BRAIN P., SAUGRAIN J. Physical features, sickling and serology of the Malagasy of Madagascar. Am J Phys Anthropol. 1957 Mar;15(1):91–124. doi: 10.1002/ajpa.1330150113. [DOI] [PubMed] [Google Scholar]
- Tuan D., Biro P. A., deRiel J. K., Lazarus H., Forget B. G. Restriction endonuclease mapping of the human gamma globin gene loci. Nucleic Acids Res. 1979 Jun 11;6(7):2519–2544. doi: 10.1093/nar/6.7.2519. [DOI] [PMC free article] [PubMed] [Google Scholar]


