Abstract
1. The effects of antagonism of the maternal renin-angiotensin system (RAS) with either an angiotensin II type 1-(AT1) specific receptor blocker (GR138950) or an angiotensin-converting enzyme (ACE) inhibitor (captopril) were compared in chronically-catheterised ewes and their foetuses during late gestation. 2. Daily from 127 +/- 1 days of gestation until parturition at 145 +/- 2 days, each ewe received i.v. either GR138950 (3 mg kg-1; n = 10), captopril (3 mg kg-1; n = 6) or an equivalent volume of vehicle solution (0.9% w/v saline; n = 10). 3. Within 2 h of drug administration, GR138950 abolished the maternal, but not the foetal, pressor responses to angiotensin II (AII; 100-188 ng kg-1, i.v.; P < 0.05), whereas captopril abolished both the maternal and foetal pressor responses to angiotensin I (AI; 400-750 ng kg-1, i.v.; P < 0.05). 4. On the first day of treatment, maternal blood pressure decreased in all GR138950-treated (-21 +/- 4 mmHg; P < 0.05) and captopril-treated (-13 +/- 5 mmHg; P > 0.05) ewes at 2 h after drug administration. Captopril also significantly decreased foetal blood pressure by 5 +/- 1 mmHg (P < 0.05). However, foetal blood pressure in the GR138950-treated animals remained unchanged. Maternal and foetal heart rates were unaffected by any treatment. Uterine blood flow was significantly reduced within 2 h of both GR138950 (-130 +/- 20 ml min-1; P < 0.05) and captopril (-72 +/- 16 ml min-1; P < 0.05) administration. 5. On the first day of treatment, maternal arterial haemoglobin (Hb) concentration and oxygen (O2) content increased at 2 h in all GR138950-treated and captopril-treated ewes. Foetal arterial pH and oxygenation (O2 content, O2 saturation and Pao2) were reduced by a similar extent in both groups of drug-treated ewes. 6. After one week of daily GR138950 administration, maternal blood pressure decreased from a pretreatment value of 96 +/- 5 mmHg on day 1 to 79 +/- 2 mmHg by day 7 (P < 0.05). Captopril treatment had no long-term effect on maternal blood pressure. Although foetal blood pressure increased by 3 +/- 1 mmHg over a week of vehicle treatment (P < 0.05), no significant differences were observed between the long-term changes in foetal blood pressure in all three groups of animals. 7. There were no long-term effects of drug administration on maternal Hb concentration or oxygenation, or on the foetal haematological parameters. However, changes in maternal PaCo2 observed in the GR138950-treated (+1.4 +/- 0.5 mmHg; P < 0.05) and captopril-treated (+3.3 +/- 1.1 mmHg; P > 0.05) ewes were significantly different from those seen in the vehicle-treated animals (P < 0.05). 8. There were no apparent adverse effects of maternal GR138950 or captopril treatment on foetal viability. 9. The present study demonstrated that administration of either GR138950 or captopril to pregnant ewes effectively blocked the maternal RAS, and caused hypotension and a decrease in uterine blood flow. However, only captopril appeared to cross the placenta to influence directly the RAS of the sheep foetus. This suggests that the fall in foetal oxygenation observed after AT1-specific receptor blockade and ACE inhibition originates primarily from changes in the maternal and/or placental vasculature. Despite these changes, neither GR138950 nor captopril were detrimental to the outcome of pregnancy when foetal blood loss was kept to a minimum.
Full text
PDF

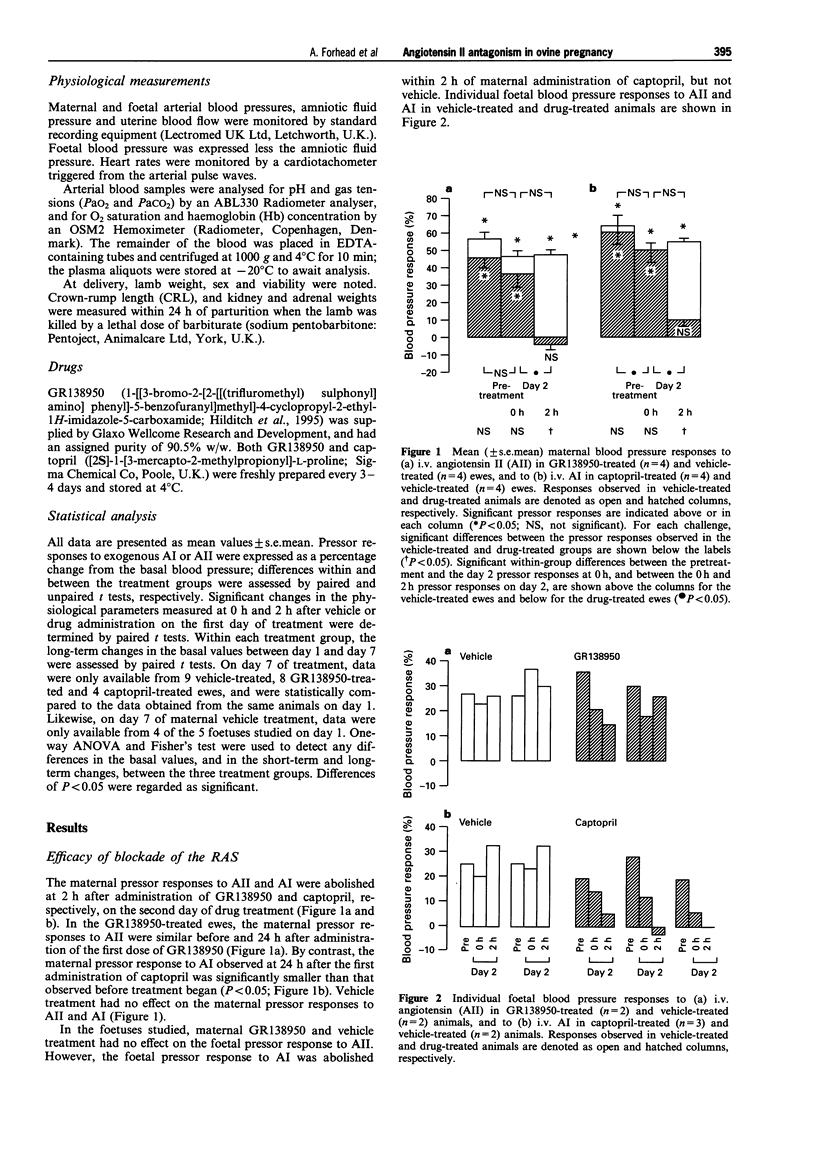
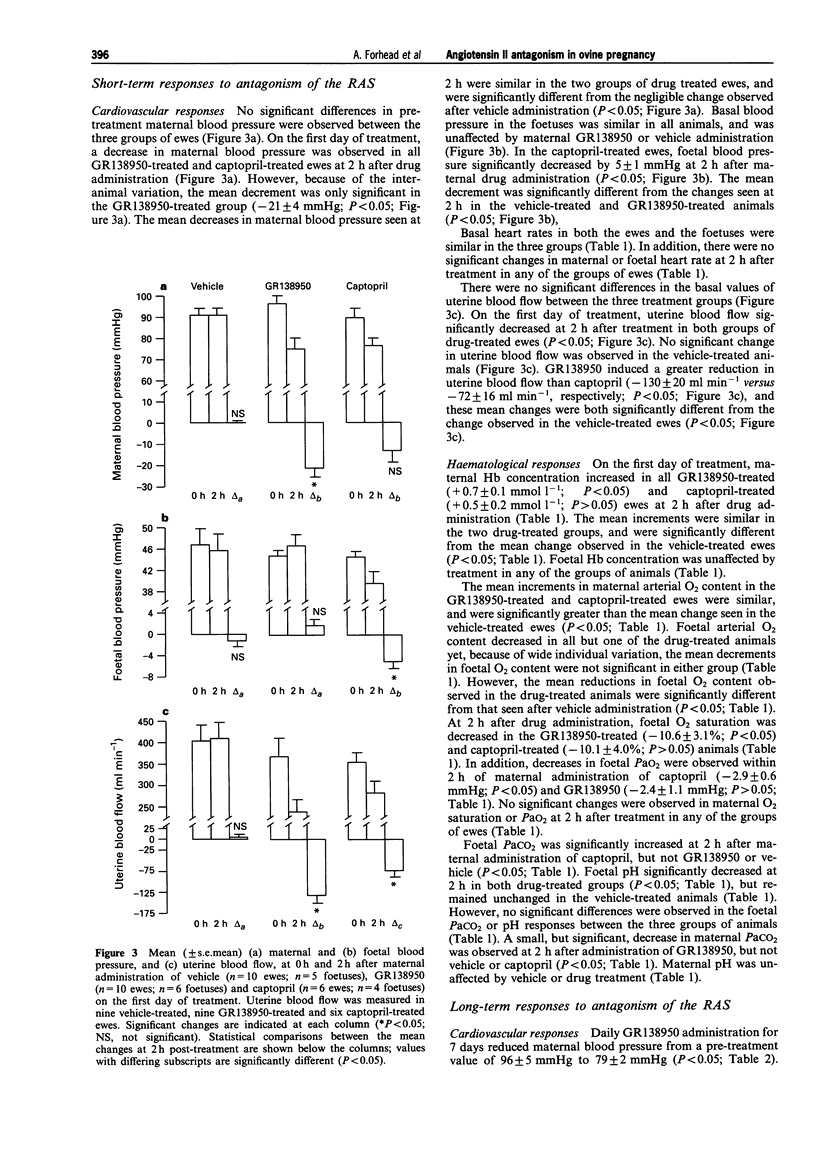
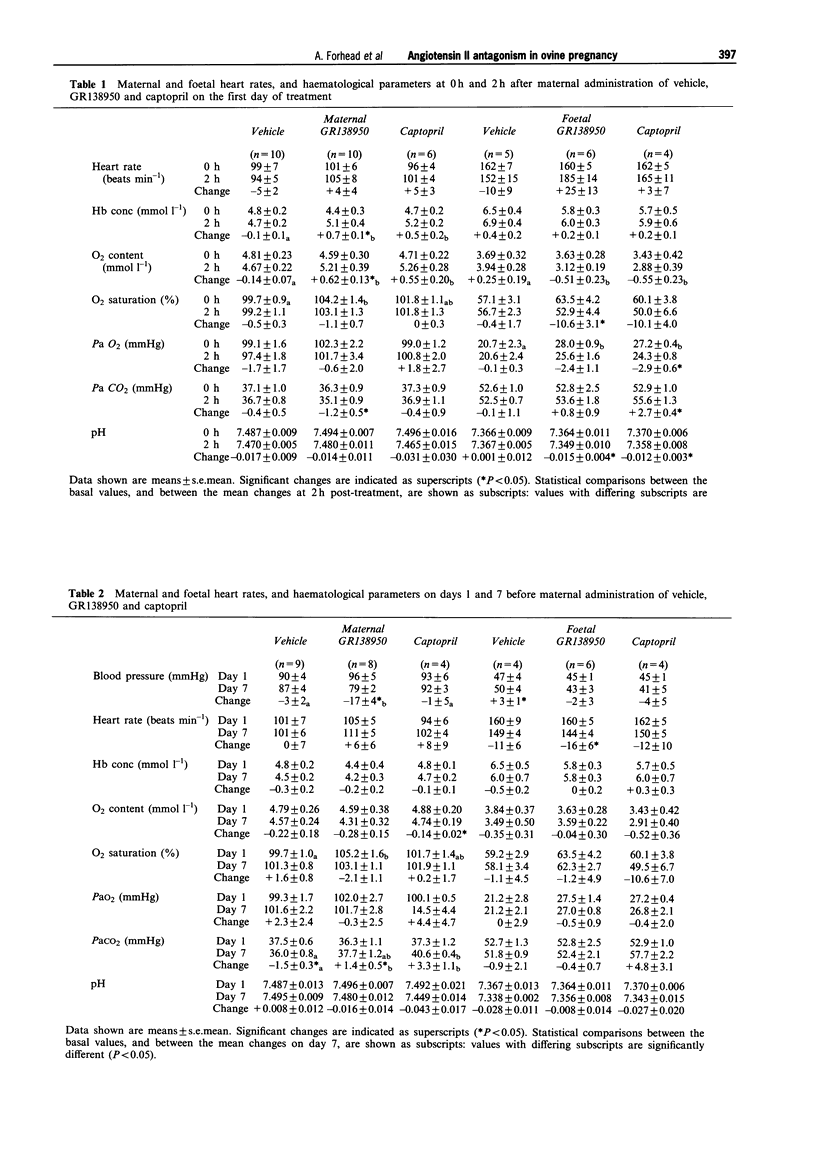




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albertini R., Seino M., Scicli A. G., Carretero O. A. Uteroplacental renin in regulation of blood pressure in the pregnant rabbit. Am J Physiol. 1980 Aug;239(2):H266–H271. doi: 10.1152/ajpheart.1980.239.2.H266. [DOI] [PubMed] [Google Scholar]
- August P., Mueller F. B., Sealey J. E., Edersheim T. G. Role of renin-angiotensin system in blood pressure regulation in pregnancy. Lancet. 1995 Apr 8;345(8954):896–897. doi: 10.1016/s0140-6736(95)90012-8. [DOI] [PubMed] [Google Scholar]
- Boddy K., Dawes G. S., Fisher R., Pinter S., Robinson J. S. Foetal respiratory movements, electrocortical and cardiovascular responses to hypoxaemia and hypercapnia in sheep. J Physiol. 1974 Dec;243(3):599–618. doi: 10.1113/jphysiol.1974.sp010768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bottari S. P., de Gasparo M., Steckelings U. M., Levens N. R. Angiotensin II receptor subtypes: characterization, signalling mechanisms, and possible physiological implications. Front Neuroendocrinol. 1993 Apr;14(2):123–171. doi: 10.1006/frne.1993.1005. [DOI] [PubMed] [Google Scholar]
- Brar H. S., Kjos S. L., Dougherty W., Do Y. S., Tam H. B., Hsueh W. A. Increased fetoplacental active renin production in pregnancy-induced hypertension. Am J Obstet Gynecol. 1987 Aug;157(2):363–367. doi: 10.1016/s0002-9378(87)80173-4. [DOI] [PubMed] [Google Scholar]
- Broughton Pipkin F., Lumbers E. R., Mott J. C. Factors influencing plasma renin and angiotensin II in the conscious pregnant ewe and its foetus. J Physiol. 1974 Dec;243(3):619–636. doi: 10.1113/jphysiol.1974.sp010769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broughton Pipkin F., O'Brien P. M. Effect of the specific angiotensin antagonist (Sar1) (Ala8) angiotensin II on blood pressure and the renin-angiotensin system in the conscious pregnant ewe and fetus. Am J Obstet Gynecol. 1978 Sep 1;132(1):7–15. doi: 10.1016/0002-9378(78)90790-1. [DOI] [PubMed] [Google Scholar]
- Broughton Pipkin F., Symonds E. M., Turner S. R. The effect of captopril (SQ14,225) upon mother and fetus in the chronically cannulated ewe and in the pregnant rabbit. J Physiol. 1982 Feb;323:415–422. doi: 10.1113/jphysiol.1982.sp014081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broughton Pipkin F., Wallace C. P. The effect of enalapril (MK421), an angiotensin converting enzyme inhibitor, on the conscious pregnant ewe and her foetus. Br J Pharmacol. 1986 Mar;87(3):533–542. doi: 10.1111/j.1476-5381.1986.tb10195.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruce S. L., Morishima H. O., Petrie R. H., Sakuma K., Daniel S. S., Yeh S. Y. Response of ovine uterine blood flow to angiotensin II: effect on the fetus. Am J Obstet Gynecol. 1981 Nov 1;141(5):495–498. doi: 10.1016/s0002-9378(15)33267-1. [DOI] [PubMed] [Google Scholar]
- Clark K. E., Irion G. L., Mack C. E. Differential responses of uterine and umbilical vasculatures to angiotensin II and norepinephrine. Am J Physiol. 1990 Jul;259(1 Pt 2):H197–H203. doi: 10.1152/ajpheart.1990.259.1.H197. [DOI] [PubMed] [Google Scholar]
- Comline R. S., Silver M. The composition of foetal and maternal blood during parturition in the ewe. J Physiol. 1972 Apr;222(1):233–256. doi: 10.1113/jphysiol.1972.sp009795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferris T. F., Weir E. K. Effect of captopril on uterine blood flow and prostaglandin E synthesis in the pregnant rabbit. J Clin Invest. 1983 Apr;71(4):809–815. doi: 10.1172/JCI110834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleischman A. R., Oakes G. K., Epstein M. F., Catt K. J., Chez R. A. Plasma renin activity during ovine pregnancy. Am J Physiol. 1975 Mar;228(3):901–904. doi: 10.1152/ajplegacy.1975.228.3.901. [DOI] [PubMed] [Google Scholar]
- Forhead A. J., Fowden A. L., Silver M., Hughes P., Broughton-Pipkin F., Sutherland M. F. Haemodynamic responses to an angiotensin II receptor antagonist (GR 117289) in maternal and fetal sheep. Exp Physiol. 1995 Mar;80(2):285–298. doi: 10.1113/expphysiol.1995.sp003848. [DOI] [PubMed] [Google Scholar]
- Gant N. F., Daley G. L., Chand S., Whalley P. J., MacDonald P. C. A study of angiotensin II pressor response throughout primigravid pregnancy. J Clin Invest. 1973 Nov;52(11):2682–2689. doi: 10.1172/JCI107462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanssens M., Keirse M. J., Vankelecom F., Van Assche F. A. Fetal and neonatal effects of treatment with angiotensin-converting enzyme inhibitors in pregnancy. Obstet Gynecol. 1991 Jul;78(1):128–135. [PubMed] [Google Scholar]
- Harewood W. J., Phippard A. F., Duggin G. G., Horvath J. S., Tiller D. J. Fetotoxicity of angiotensin-converting enzyme inhibition in primate pregnancy: a prospective, placebo-controlled study in baboons (Papio hamadryas). Am J Obstet Gynecol. 1994 Sep;171(3):633–642. doi: 10.1016/0002-9378(94)90075-2. [DOI] [PubMed] [Google Scholar]
- Hilditch A., Hunt A. A., Travers A., Polley J., Drew G. M., Middlemiss D., Judd D. B., Ross B. C., Robertson M. J. Pharmacological effects of GR138950, a novel angiotensin AT1 receptor antagonist. J Pharmacol Exp Ther. 1995 Feb;272(2):750–757. [PubMed] [Google Scholar]
- Ito M., Nakamura T., Yoshimura T., Koyama H., Okamura H. The blood pressure response to infusions of angiotensin II during normal pregnancy: relation to plasma angiotensin II concentration, serum progesterone level, and mean platelet volume. Am J Obstet Gynecol. 1992 Apr;166(4):1249–1253. doi: 10.1016/s0002-9378(11)90617-6. [DOI] [PubMed] [Google Scholar]
- Iwamoto H. S., Rudolph A. M. Effects of endogenous angiotensin II on the fetal circulation. J Dev Physiol. 1979 Aug;1(4):283–293. [PubMed] [Google Scholar]
- Iwamoto H. S., Rudolph A. M. Role of renin-angiotensin system in response to hemorrhage in fetal sheep. Am J Physiol. 1981 Jun;240(6):H848–H854. doi: 10.1152/ajpheart.1981.240.6.H848. [DOI] [PubMed] [Google Scholar]
- Kalenga M. K., de Gasparo M., de Hertogh R., Whitebread S., Vankrieken L., Thomas K. Les récepteurs de l'angiotensine II dans le placenta humain sont de type AT1. Reprod Nutr Dev. 1991;31(3):257–267. [PubMed] [Google Scholar]
- Loquet P., Broughton Pipkin F., Symonds E. M., Rubin P. C. Influence of raising maternal blood pressure with angiotensin II on utero-placental and feto-placental blood velocity indices in the human. Clin Sci (Lond) 1990 Jan;78(1):95–100. doi: 10.1042/cs0780095. [DOI] [PubMed] [Google Scholar]
- Lumbers E. R., Burrell J. H., Menzies R. I., Stevens A. D. The effects of a converting enzyme inhibitor (captopril) and angiotensin II on fetal renal function. Br J Pharmacol. 1993 Oct;110(2):821–827. doi: 10.1111/j.1476-5381.1993.tb13886.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lumbers E. R., Kingsford N. M., Menzies R. I., Stevens A. D. Acute effects of captopril, an angiotensin-converting enzyme inhibitor, on the pregnant ewe and fetus. Am J Physiol. 1992 May;262(5 Pt 2):R754–R760. doi: 10.1152/ajpregu.1992.262.5.R754. [DOI] [PubMed] [Google Scholar]
- MacFadyen R. J., Reid J. L. Angiotensin receptor antagonists as a treatment for hypertension. J Hypertens. 1994 Dec;12(12):1333–1338. [PubMed] [Google Scholar]
- Mackanjee H. R., Shaul P. W., Magness R. R., Rosenfeld C. R. Angiotensin II vascular smooth-muscle receptors are not down-regulated in near-term pregnant sheep. Am J Obstet Gynecol. 1991 Dec;165(6 Pt 1):1641–1648. doi: 10.1016/0002-9378(91)90008-f. [DOI] [PubMed] [Google Scholar]
- Olsson K., Fyhrquist F., Benlamlih S., Dahlborn K. Effects of captopril on arterial blood pressure, plasma renin activity and vasopressin concentration in sodium-repleted and sodium-deficient goats. A serial study during pregnancy, lactation and anestrus. Acta Physiol Scand. 1984 May;121(1):73–80. doi: 10.1111/j.1748-1716.1984.tb10459.x. [DOI] [PubMed] [Google Scholar]
- Rankin J. H., Phernetton T. M. Alpha and angiotensin receptor tone in the near-term sheep fetus. Proc Soc Exp Biol Med. 1978 Jun;158(2):166–169. doi: 10.3181/00379727-158-40163. [DOI] [PubMed] [Google Scholar]
- Robillard J. E., Weismann D. N., Gomez R. A., Ayres N. A., Lawton W. J., VanOrden D. E. Renal and adrenal responses to converting-enzyme inhibition in fetal and newborn life. Am J Physiol. 1983 Feb;244(2):R249–R256. doi: 10.1152/ajpregu.1983.244.2.R249. [DOI] [PubMed] [Google Scholar]
- Rosenfeld C. R., Gant N. F., Jr The chronically instrumental ewe: a model for studying vascular reactivity to angiotensin II in pregnancy. J Clin Invest. 1981 Feb;67(2):486–492. doi: 10.1172/JCI110057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shanmugam S., Monnot C., Corvol P., Gasc J. M. Distribution of type 1 angiotensin II receptor subtype messenger RNAs in the rat fetus. Hypertension. 1994 Jan;23(1):137–141. doi: 10.1161/01.hyp.23.1.137. [DOI] [PubMed] [Google Scholar]
- Stevenson K. M., Gibson K. J., Lumbers E. R. Comparison of the transplacental transfer of enalapril, captopril and losartan in sheep. Br J Pharmacol. 1995 Apr;114(7):1495–1501. doi: 10.1111/j.1476-5381.1995.tb13376.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timmermans P. B., Chiu A. T., Herblin W. F., Wong P. C., Smith R. D. Angiotensin II receptor subtypes. Am J Hypertens. 1992 Jun;5(6 Pt 1):406–410. doi: 10.1093/ajh/5.6.406. [DOI] [PubMed] [Google Scholar]
- Tsutsumi K., Strömberg C., Viswanathan M., Saavedra J. M. Angiotensin-II receptor subtypes in fetal tissue of the rat: autoradiography, guanine nucleotide sensitivity, and association with phosphoinositide hydrolysis. Endocrinology. 1991 Aug;129(2):1075–1082. doi: 10.1210/endo-129-2-1075. [DOI] [PubMed] [Google Scholar]
- Woods L. L. Role of angiotensin II and prostaglandins in the regulation of uteroplacental blood flow. Am J Physiol. 1993 Mar;264(3 Pt 2):R584–R590. doi: 10.1152/ajpregu.1993.264.3.R584. [DOI] [PubMed] [Google Scholar]


