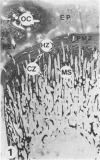
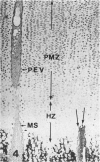
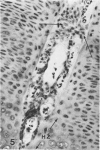
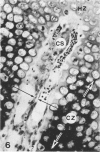
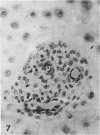
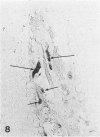
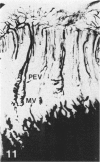
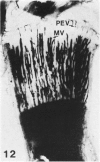
Abstract
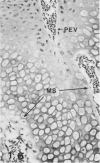
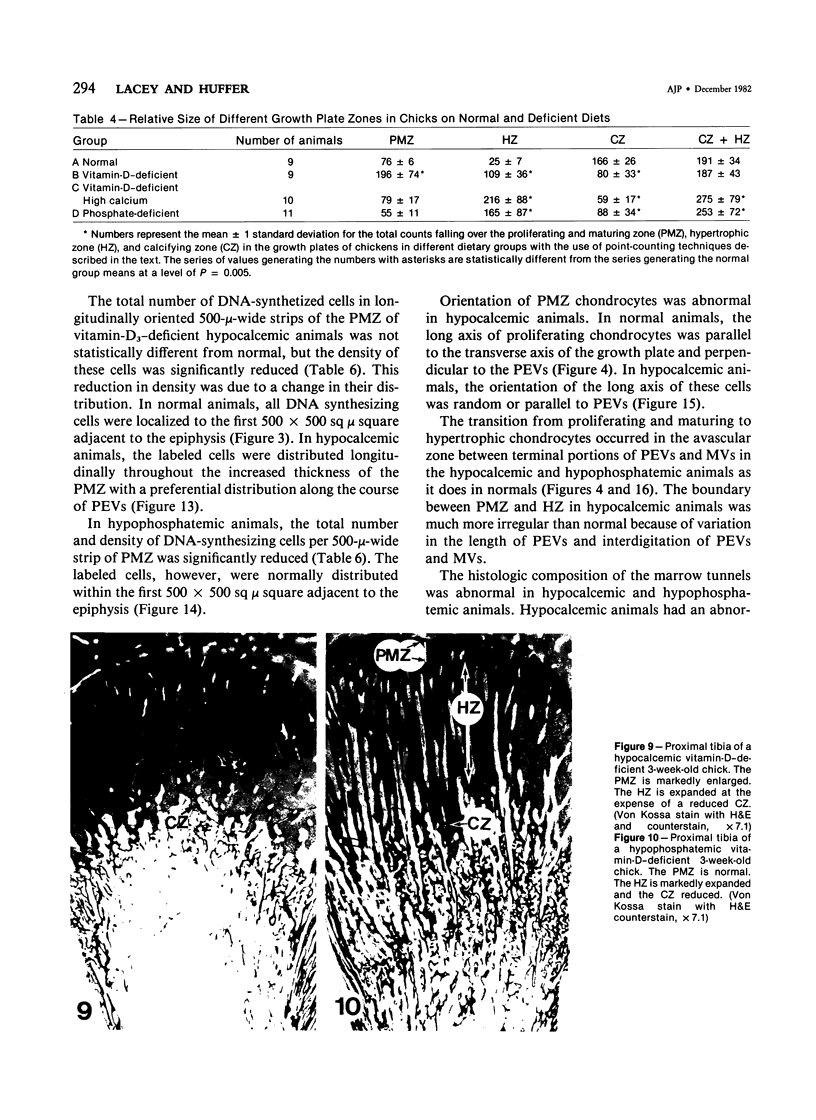
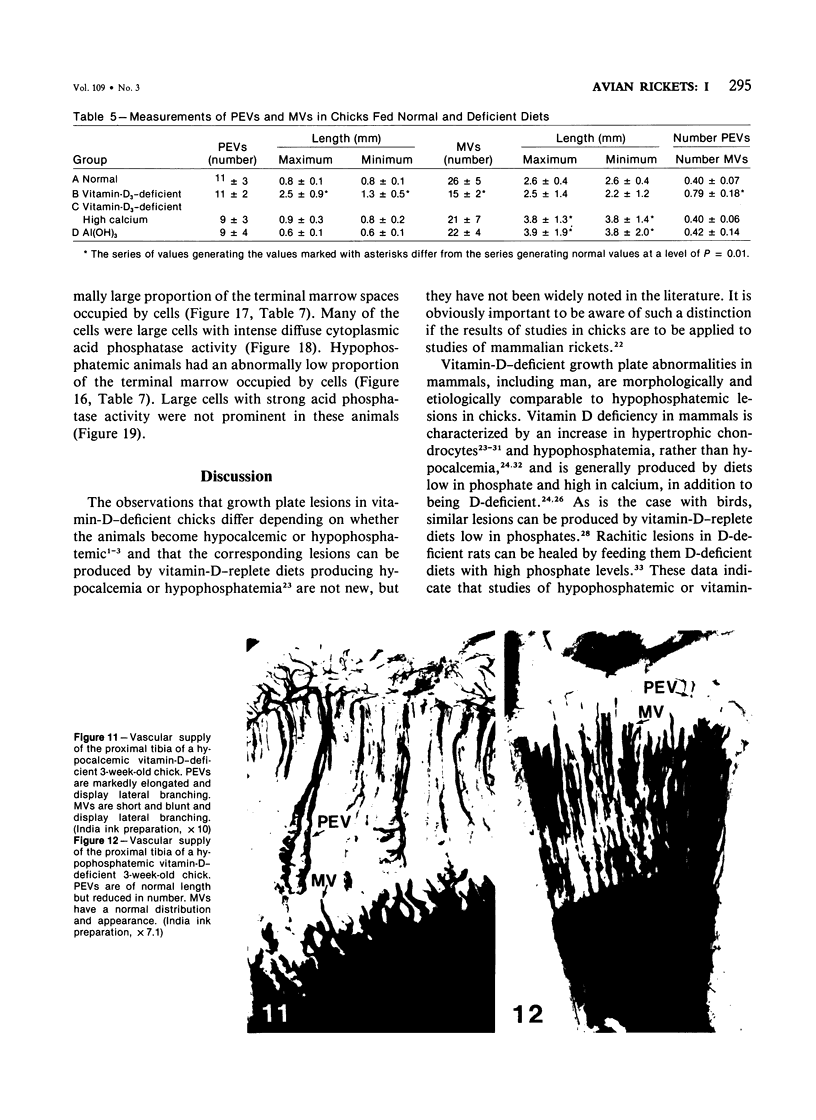
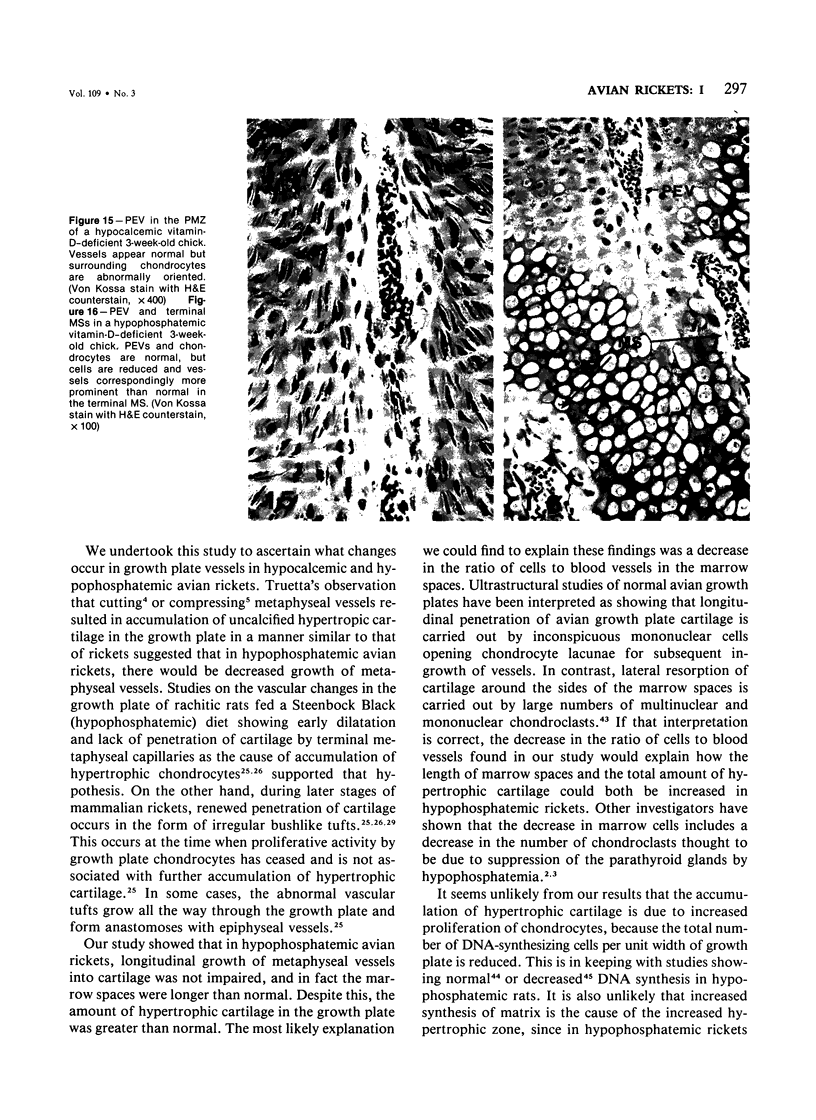
Growth plate morphometry and measurements of serum chemistry were correlated to clarify the pathogenesis of hypocalcemic and hypophosphatemic avian rickets. Accumulation of proliferating and maturing cartilage in hypocalcemic chicks is accompanied by increased length and increased variation in length of perforating epiphyseal vessels, decreased number and abnormal arrangement of marrow spaces, an increased proportion of cells to blood vessels in marrow spaces, and a change in the distribution but not the total number of DNA-synthesizing chondrocytes per unit width of growth plate. Accumulation of hypertrophic cartilage in hypophosphatemic rickets is accompanied by no change in length, distribution, or number of perforating epiphyseal vessels, elongation but no change in number or arrangement of marrow spaces, an increase in the relative proportion of blood vessels to cells in the marrow spaces, and no change in distribution but a decrease in total number of DNA-synthesizing chondrocytes per unit width of growth plate. Both types of rickets have decreased amounts of calcified cartilage. These results provide further evidence that hypocalcemia and hypophosphatemia cause morphologically distinct types of rickets in birds. The data indicate that the thickness of the proliferating and maturing region and hence the distance of the hypertrophic zones from the epiphysis are anatomically and temporally related to length of perforating epiphyseal vessels and serum calcium levels. They indicate that in hypocalcemic rickets accumulation of proliferating and maturing cartilage is unlikely to be the result of increased chondrocyte replication and that the relative rates of chondrocyte hypertrophy and resorption of hypertrophic cartilage by marrow are equal. They support the concept that delayed chondrocyte hypertrophy is the major cause of growth plate thickening in hypocalcemic rickets. Data presented in this study, when considered together with data from the literature on hypophosphatemic rickets, support the long-held concept that growth plate thickening in this disease is caused primarily by a decreased rate of resorption of hypertrophic cartilage by marrow relative to the rates of chondrocyte proliferation, maturation, and hypertrophy. The data further support the concepts that growth of cartilage into marrow is a biphasic process including longitudinal growth effected mainly by blood vessels, and resorption of the lateral walls of marrow spaces effected mainly by marrow cells, and that it is the latter phase that is defective in hypophosphatemia.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beaumont G. D. The intraosseous vasculature of the ulna of Gallus domesticus. J Anat. 1967 Jun;101(Pt 3):543–554. [PMC free article] [PubMed] [Google Scholar]
- Bisaz S., Schenk R., Kunin A. S., Russell R. G., Mühlbauer R., Fleisch H. The comparative effects of vitamin d deficiency and ethane-1-hydroxy-1,1-diphosphonate administration on the histology and glycolysis of chick epiphyseal and articular cartilage. Calcif Tissue Res. 1975 Dec 18;19(2):139–152. doi: 10.1007/BF02563998. [DOI] [PubMed] [Google Scholar]
- FOLLIS R. H., Jr, PARK E. A., JACKSON D. The prevalence of rickets at autopsy during the first two years of age. Bull Johns Hopkins Hosp. 1952 Dec;91(6):480–497. [PubMed] [Google Scholar]
- Garner A., Ball J. Quantitative observations on mineralised and unmineralised bone in chronic renal azotaemia and intestinal malabsorption syndrome. J Pathol Bacteriol. 1966 Apr;91(2):545–561. doi: 10.1002/path.1700910231. [DOI] [PubMed] [Google Scholar]
- Graham R. C., Jr, Karnovsky M. J. The early stages of absorption of injected horseradish peroxidase in the proximal tubules of mouse kidney: ultrastructural cytochemistry by a new technique. J Histochem Cytochem. 1966 Apr;14(4):291–302. doi: 10.1177/14.4.291. [DOI] [PubMed] [Google Scholar]
- Groth W., Frey H. Zur Differenzierung der Wirkung eines Mangels and Calcium, Phosphor oder Vitamin D auf Knochen, Blut und innersekretorische Drüsen des Hühnerkükens. Zentralbl Veterinarmed A. 1966 Jun;13(4):302–319. [PubMed] [Google Scholar]
- HJERTQUIST S. O. Autoradiographic study of the proximal tibial growth zone in normal and rachitic rats after administration of radiosulphate. Acta Pathol Microbiol Scand Suppl. 1962;Suppl 154:99–100. [PubMed] [Google Scholar]
- HURST R. O. THE DETERMINATION OF NUCLEOTIDE PHOSPHORUS WITH A STANNOUS CHLORIDE-HYDRAZINE SULPHATE REAGENT. Can J Biochem. 1964 Feb;42:287–292. doi: 10.1139/o64-033. [DOI] [PubMed] [Google Scholar]
- Harrison J. E., Hitchman A. J., HItchman A., Hasany S. A., McNeill K. G., Tam C. S. Differences between the effects of phosphate deficiency and vitamin D deficiency on bone metabolism. Metabolism. 1980 Dec;29(12):1225–1233. doi: 10.1016/0026-0495(80)90149-3. [DOI] [PubMed] [Google Scholar]
- Howlett C. R. The fine structure of the proximal growth plate and metaphysis of the avian tibia: endochondral osteogenesis. J Anat. 1980 Jun;130(Pt 4):745–768. [PMC free article] [PubMed] [Google Scholar]
- Hunt C. D., Ollerich D. A., Nielsen F. H. Morphology of the perforating cartilage canals in the proximal tibial growth plate of the chick. Anat Rec. 1979 May;194(1):143–157. doi: 10.1002/ar.1091940110. [DOI] [PubMed] [Google Scholar]
- IRVING M. H. THE BLOOD SUPPLY OF THE GROWTH CARTILAGE AND METAPHYSIS IN RACHITIC RATS. J Pathol Bacteriol. 1965 Apr;89:461–471. doi: 10.1002/path.1700890203. [DOI] [PubMed] [Google Scholar]
- Jande S. S., Dickson I. R. Comparative histological study of the effects of high calcium diet and vitamin D supplements on epiphyseal plates of vitamin-D-deficient chicks. Acta Anat (Basel) 1980;108(4):463–468. doi: 10.1159/000145345. [DOI] [PubMed] [Google Scholar]
- Kooh S. W., Fraser D., Reilly B. J., Hamilton J. R., Gall D. G., Bell L. Rickets due to calcium deficiency. N Engl J Med. 1977 Dec 8;297(23):1264–1266. doi: 10.1056/NEJM197712082972307. [DOI] [PubMed] [Google Scholar]
- Krempien B., Mehls O., Ritz E. Morphological studies on pathogenesis of epiphyseal slipping in uremic children. Virchows Arch A Pathol Anat Histol. 1974 Jan 30;362(2):129–143. doi: 10.1007/BF00432391. [DOI] [PubMed] [Google Scholar]
- Lapatsanis P., Makaronis G., Vretos C., Doxiadis S. Two types of nutritional rickets in infants. Am J Clin Nutr. 1976 Nov;29(11):1222–1226. doi: 10.1093/ajcn/29.11.1222. [DOI] [PubMed] [Google Scholar]
- Lutfi A. M. Mode of growth, fate and functions of cartilage canals. J Anat. 1970 Jan;106(Pt 1):135–145. [PMC free article] [PubMed] [Google Scholar]
- Mankin H. J., Lippiello L. Nucleic acid and protein synthesis in epiphyseal plates of rachitic rats. An autoradiographic study. J Bone Joint Surg Am. 1969 Jul;51(5):862–874. [PubMed] [Google Scholar]
- Mehls O., Ritz E., Gilli G., Schmidt-Gayk H., Krempien B., Kourist B., Wesch H., Prager P. Skeletal changes and growth in experimental uremia. Nephron. 1977;18(5):288–300. doi: 10.1159/000180845. [DOI] [PubMed] [Google Scholar]
- Mehls O., Ritz E., Gilli G., Wangdak T., Krempien B. Effect of vitamin D on growth in experimental uremia. Am J Clin Nutr. 1978 Oct;31(10):1927–1931. doi: 10.1093/ajcn/31.10.1927. [DOI] [PubMed] [Google Scholar]
- Mehls O., Ritz E., Krempien B., Gilli G., Link K., Willich E., Schärer K. Slipped epiphyses in renal osteodystrophy. Arch Dis Child. 1975 Jul;50(7):545–554. doi: 10.1136/adc.50.7.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen P. The action of vitamin D deficiency on bone tissue and the epiphyseal plate in rats given adequate amounts of calcium and phosphorus in the diet. Arch Oral Biol. 1969 Nov;14(11):1293–1304. doi: 10.1016/0003-9969(69)90202-7. [DOI] [PubMed] [Google Scholar]
- Simmons D. J., Kunin A. S. Development and healing of rickets in rats. I. Studies with tritiated thymidine and nutritional considerations. Clin Orthop Relat Res. 1970 Jan-Feb;68:251–260. [PubMed] [Google Scholar]
- Simmons D. J., Kunin A. S. Development and healing of rickets in rats. II. Studies with tritiated proline. Clin Orthop Relat Res. 1970 Jan-Feb;68:261–272. [PubMed] [Google Scholar]
- Stefanini M., De Martino C., Zamboni L. Fixation of ejaculated spermatozoa for electron microscopy. Nature. 1967 Oct 14;216(5111):173–174. doi: 10.1038/216173a0. [DOI] [PubMed] [Google Scholar]
- TRUETA J., BUHR A. J. THE VASCULAR CONTRIBUTION TO OSTEOGENESIS. V. THE VASCULATURE SUPPLYING THE EPIPHYSIAL CARTILAGE IN RACHITIC RATS. J Bone Joint Surg Br. 1963 Aug;45:572–581. [PubMed] [Google Scholar]
- TRUETA J., LITTLE K. The vascular contribution to osteogenesis. II. Studies with the electron microscope. J Bone Joint Surg Br. 1960 May;42-B:367–376. doi: 10.1302/0301-620X.42B2.367. [DOI] [PubMed] [Google Scholar]
- TRUETA J., MORGAN J. D. The vascular contribution to osteogenesis. I. Studies by the injection method. J Bone Joint Surg Br. 1960 Feb;42-B:97–109. doi: 10.1302/0301-620X.42B1.97. [DOI] [PubMed] [Google Scholar]
- TRUETA J., TRIAS A. The vascular contribution to osteogenesis. IV. The effect of pressure upon the epiphysial cartilage of the rabbit. J Bone Joint Surg Br. 1961 Nov;43-B:800–813. doi: 10.1302/0301-620X.43B4.800. [DOI] [PubMed] [Google Scholar]
- Trueta J., Amato V. P. The vascular contribution to osteogenesis. III. Changes in the growth cartilage caused by experimentally induced ischaemia. J Bone Joint Surg Br. 1960 Aug;42-B:571–587. doi: 10.1302/0301-620X.42B3.571. [DOI] [PubMed] [Google Scholar]
- Wise D. R., Jennings A. R. The development and morphology of the growth plates of two long bones of the turkey. Res Vet Sci. 1973 Mar;14(2):161–166. [PubMed] [Google Scholar]
- ZETTNER A., SELIGSON D. APPLICATION OF ATOMIC ABSORPTION SPECTROPHOTOMETRY IN THE DETERMINATION OF CALCIUM IN SERUM. Clin Chem. 1964 Oct;10:869–890. [PubMed] [Google Scholar]