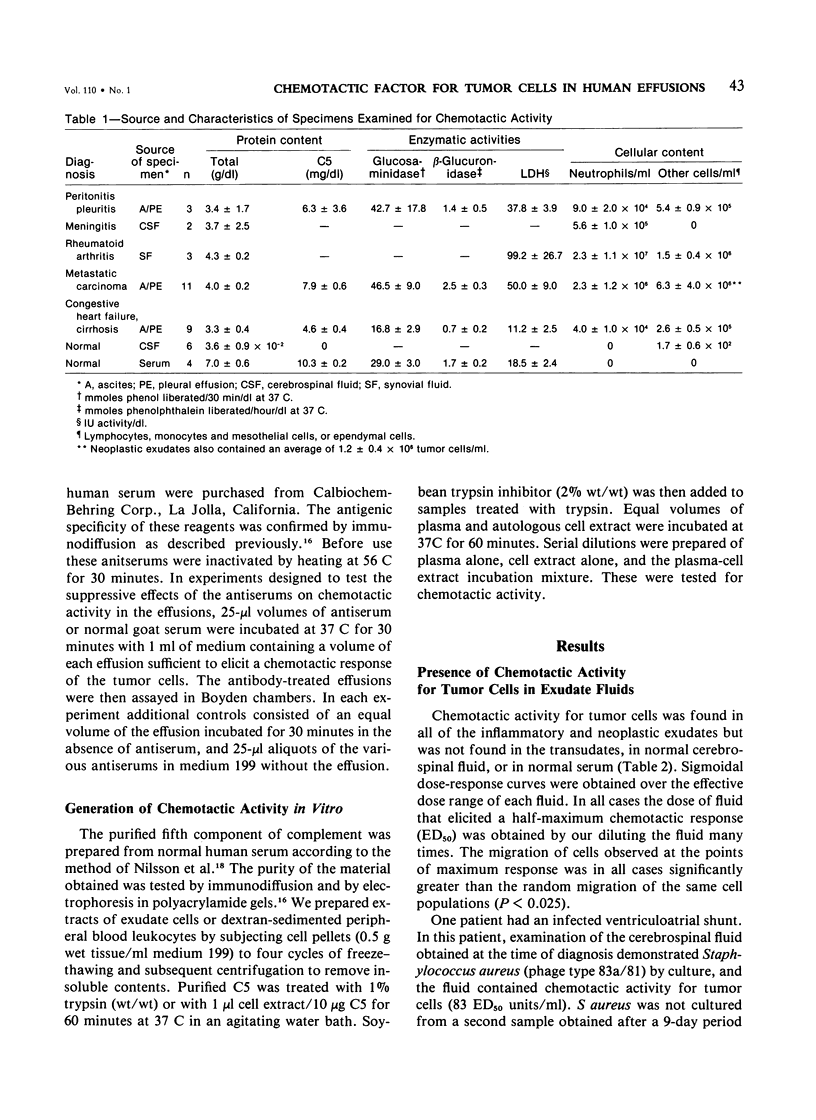
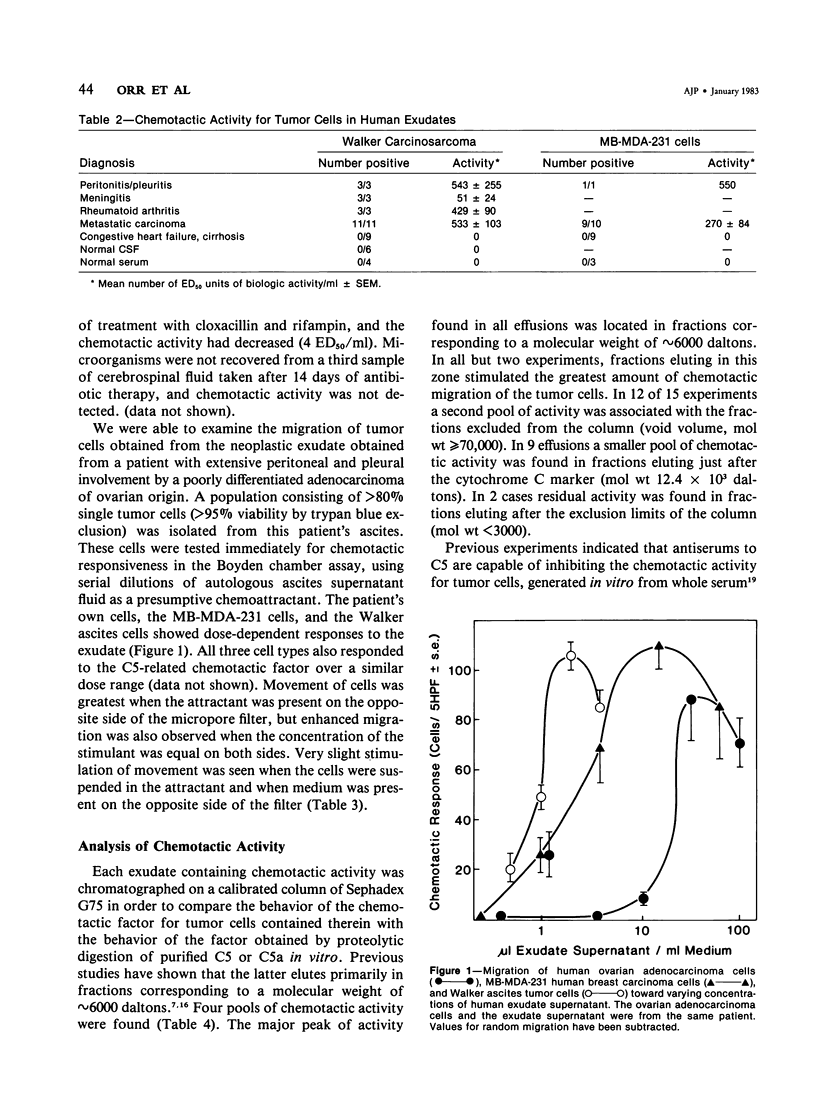
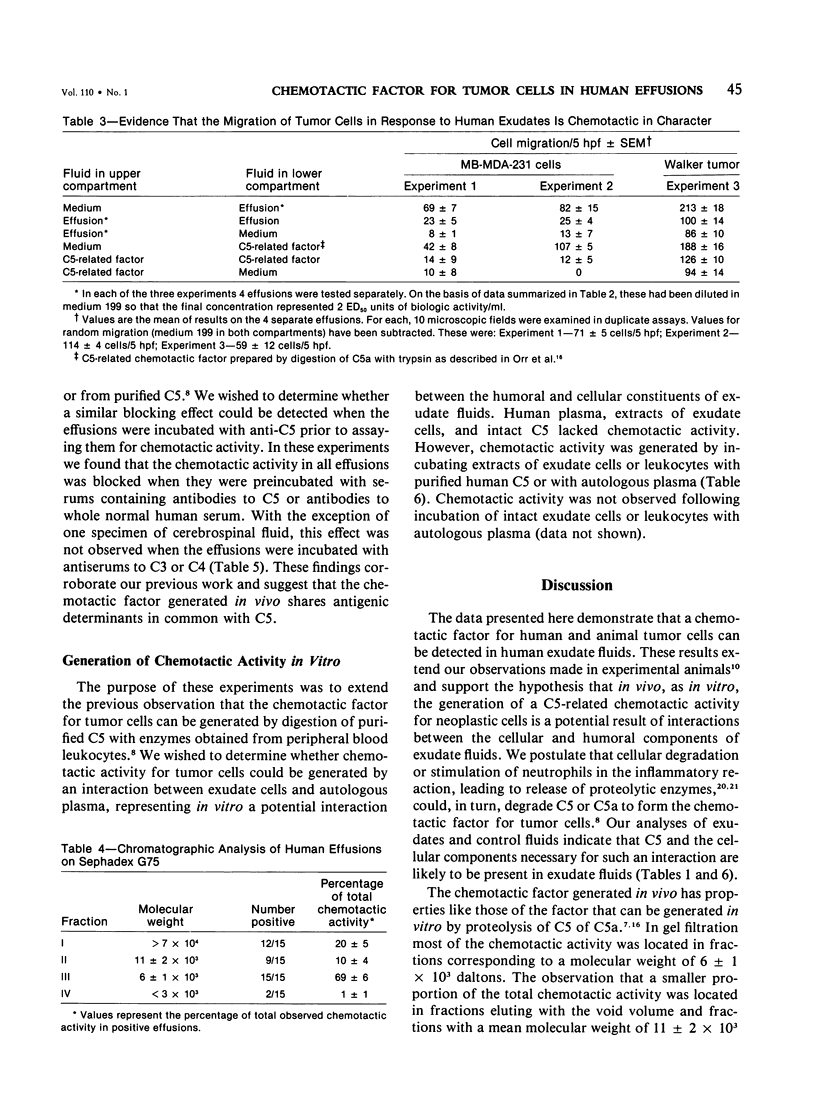
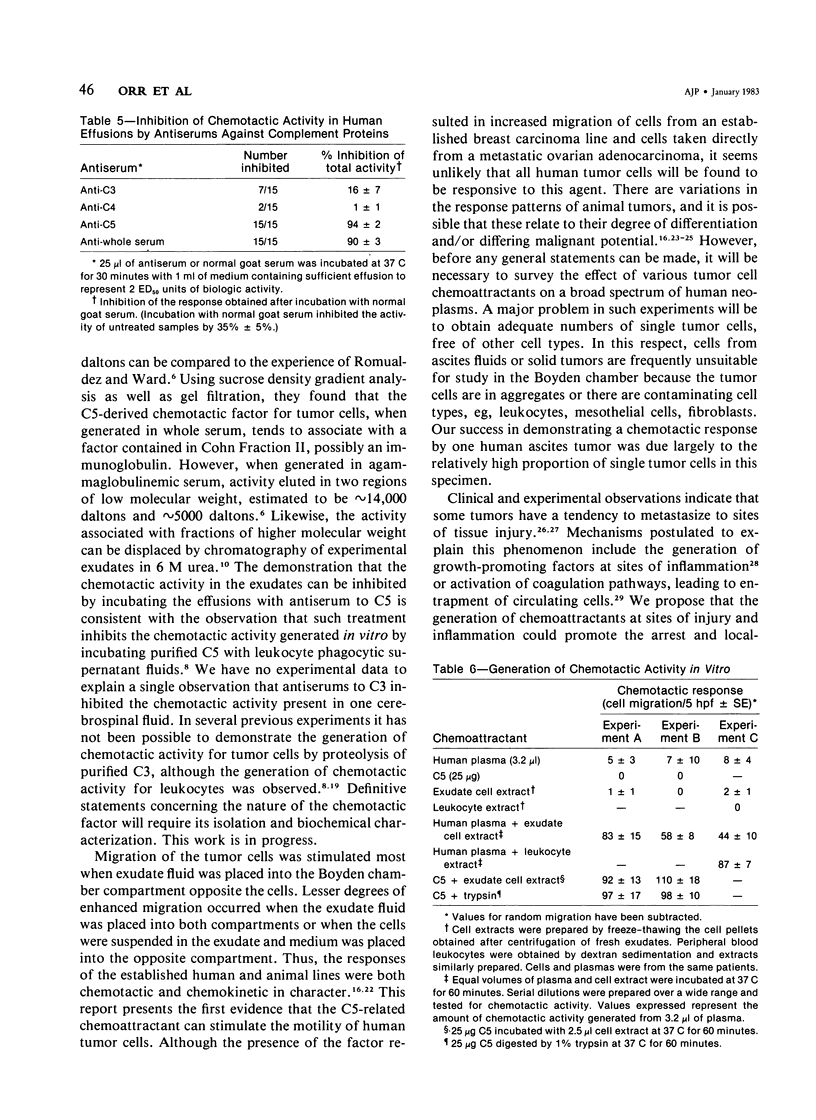
Abstract
A chemotactic factor for neoplastic cells can be generated in vitro by incubating human C5 or C5a with leukocytic or pancreatic lysosomal enzymes and is also detectable in experimental inflammatory exudates. The authors therefore sought evidence for the existence of this factor in human effusions. Using the Boyden chamber assay, they detected chemotactic activity for MB-MDA-231 human breast carcinoma cells and Walker ascites tumor cells in human inflammatory and neoplastic exudates, including ascites, pleural effusions, synovial fluids and cerebrospinal fluids. Chemotactic activity was not found in transudates, normal cerebrospinal fluid, or normal serum. Human ovarian adenocarcinoma cells from one of the effusions migrated toward autologous ascites and towards the C5-derived chemotactic factor that had been prepared in vitro. In gel filtration the chemotactic factor behaved generally as a molecule having a molecular weight of approximately 6000 daltons. The activity was blocked after incubation with antiserums directed against C5 but not by antiserums directed against C3 or C4. In vitro, chemotactic activity for tumor cells could be generated by incubating extracts of exudate cells with autologous plasma or with purified C5. The authors conclude that a chemotactic factor for tumor cells can be formed in human effusions and that this factor has properties similar to those of a previously described C5-derived chemotactic factor.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Becker E. L., Showell H. J., Henson P. M., Hsu L. S. The ability of chemotactic factors to induce lysosomal enzyme release. I. The characteristics of the release, the importance of surfaces and the relation of enzyme release to chemotactic responsiveness. J Immunol. 1974 Jun;112(6):2047–2054. [PubMed] [Google Scholar]
- Bosmann H. B., Lockwood T., Morgan H. R. Surface biochemical changes accompanying primary infection with Rous sarcoma virus. II. Proteolytic and glycosidase activity and sublethal autolysis. Exp Cell Res. 1974 Jan;83(1):25–30. doi: 10.1016/0014-4827(74)90683-1. [DOI] [PubMed] [Google Scholar]
- Cailleau R., Young R., Olivé M., Reeves W. J., Jr Breast tumor cell lines from pleural effusions. J Natl Cancer Inst. 1974 Sep;53(3):661–674. doi: 10.1093/jnci/53.3.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DerHagopian R. P., Sugarbaker E. V., Ketcham A. Inflammatory oncotaxis. JAMA. 1978 Jul 28;240(4):374–375. [PubMed] [Google Scholar]
- Fisher B., Fisher E. R., Feduska N. Trauma and the localization of tumor cells. Cancer. 1967 Jan;20(1):23–30. doi: 10.1002/1097-0142(1967)20:1<23::aid-cncr2820200103>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- Goldstein I. M., Weissmann G. Generation of C5-derived lysosomal enzyme-releasing activity (C5a) by lysates of leukocyte lysosomes. J Immunol. 1974 Nov;113(5):1583–1588. [PubMed] [Google Scholar]
- Hayashi H., Yoshida K., Ozaki T., Ushijima K. Chemotactic factor associated with invasion of cancer cells. Nature. 1970 Apr 11;226(5241):174–175. doi: 10.1038/226174a0. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lam W. C., Delikatny E. J., Orr F. W., Wass J., Varani J., Ward P. A. The chemotactic response of tumor cells. A model for cancer metastasis. Am J Pathol. 1981 Jul;104(1):69–76. [PMC free article] [PubMed] [Google Scholar]
- MUSA B. U., DOE R. P., SEAL U. S. PURIFICATION AND PROPERTIES OF HUMAN LIVER BETA-GLUCURONIDASE. J Biol Chem. 1965 Jul;240:2811–2816. [PubMed] [Google Scholar]
- Mancini G., Carbonara A. O., Heremans J. F. Immunochemical quantitation of antigens by single radial immunodiffusion. Immunochemistry. 1965 Sep;2(3):235–254. doi: 10.1016/0019-2791(65)90004-2. [DOI] [PubMed] [Google Scholar]
- Nilsson U. R., Tomar R. H., Taylor F. B., Jr Additional studies on human C5: development of a modified purification method and characterization of the purified product by polyacrylamide gel electrophoresis. Immunochemistry. 1972 Jul;9(7):709–723. doi: 10.1016/0019-2791(72)90015-8. [DOI] [PubMed] [Google Scholar]
- Orr F. W., Lam W. C., Delikatny E. J., Mokashi S., Varani J. Localization of intravenously injected tumor cells in the rat mesentery after intraperitoneal administration of chemotactic stimuli. Invasion Metastasis. 1981;1(4):239–247. [PubMed] [Google Scholar]
- Orr F. W., Mokashi S., Delikatny J. Generation of a complement-derived chemotactic factor for tumor cells in experimentally induced peritoneal exudates and its effect on the local metastasis of circulating tumor cells. Am J Pathol. 1982 Jul;108(1):112–118. [PMC free article] [PubMed] [Google Scholar]
- Orr F. W., Varani J., Delikatny J., Jain N., Ward P. A. Comparison of the chemotactic responsiveness of two fibrosarcoma subpopulations of differing malignancy. Am J Pathol. 1981 Feb;102(2):160–167. [PMC free article] [PubMed] [Google Scholar]
- Orr F. W., Varani J., Kreutzer D. L., Senior R. M., Ward P. A. Digestion of the fifth component of complement by leukocyte enzymes. Sequential generation of chemotactic activities for leukocytes and for tumor cells. Am J Pathol. 1979 Jan;94(1):75–83. [PMC free article] [PubMed] [Google Scholar]
- Orr W., Phan S. H., Varani J., Ward P. A., Kreutzer D. L., Webster R. O., Henson P. M. Chemotactic factor for tumor cells derived from the C5a fragment of complement component C5. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1986–1989. doi: 10.1073/pnas.76.4.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orr W., Varani J., Gondex M. K., Ward P. A., Mundy G. R. Chemotactic responses of tumor cells to products of resorbing bone. Science. 1979 Jan 12;203(4376):176–179. doi: 10.1126/science.569363. [DOI] [PubMed] [Google Scholar]
- Orr W., Varani J., Ward P. A. Characteristics of the chemotactic response of neoplastic cells to a factor derived from the fifth component of complement. Am J Pathol. 1978 Nov;93(2):405–422. [PMC free article] [PubMed] [Google Scholar]
- Ozaki T., Yoshida K., Ushijima K., Hayashi H. Studies on the mechanisms of invasion in cancer. II. In vivo effects of a factor chemotactic for cancer cells. Int J Cancer. 1971 Jan 15;7(1):93–100. doi: 10.1002/ijc.2910070111. [DOI] [PubMed] [Google Scholar]
- Romualdez A. G., Jr, Ward P. A. A unique complement derived chemotactic factor for tumor cells. Proc Natl Acad Sci U S A. 1975 Oct;72(10):4128–4132. doi: 10.1073/pnas.72.10.4128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romualdez A. G., Ward P. A. Further studies on the C5-derived chemotactic factor for tumor cells. Prog Clin Biol Res. 1976;9:65–71. [PubMed] [Google Scholar]
- Skolnik G., Alpsten M., Ivarsson L. Studies on mechanisms involved in metastasis formation from circulating tumor cells. Factors influencing tumor cell lodgement during normal and post-traumatic conditions. J Cancer Res Clin Oncol. 1980;97(3):249–256. doi: 10.1007/BF00405776. [DOI] [PubMed] [Google Scholar]
- Van Den Brenk H. A., Stone M., Kelly H., Orton C., Sharpington C. Promotion of growth of tumour cells in acutely inflamed tissues. Br J Cancer. 1974 Sep;30(3):246–260. doi: 10.1038/bjc.1974.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WROBLEWSKI F., LADUE J. S. Lactic dehydrogenase activity in blood. Proc Soc Exp Biol Med. 1955 Oct;90(1):210–213. doi: 10.3181/00379727-90-21985. [DOI] [PubMed] [Google Scholar]
- Ward P. A., Zvaifler N. J. Complement-derived leukotactic factors in inflammatory synovial fluids of humans. J Clin Invest. 1971 Mar;50(3):606–616. doi: 10.1172/JCI106531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wass J. A., Varani J., Piontek G. E., Goff D., Ward P. A. Characteristics of the chemotactic factor-mediated cell swelling response of tumor cells. J Natl Cancer Inst. 1981 May;66(5):927–933. [PubMed] [Google Scholar]
- Wass J. A., Varani J., Piontek G. E., Ward P. A., Orr F. W. Responses of normal and malignant cells to collagen, collagen-derived peptides and the C5-related tumor cell chemotactic peptide. Cell Differ. 1981 Dec;10(6):329–332. doi: 10.1016/0045-6039(81)90024-5. [DOI] [PubMed] [Google Scholar]
- Zigmond S. H., Hirsch J. G. Leukocyte locomotion and chemotaxis. New methods for evaluation, and demonstration of a cell-derived chemotactic factor. J Exp Med. 1973 Feb 1;137(2):387–410. doi: 10.1084/jem.137.2.387. [DOI] [PMC free article] [PubMed] [Google Scholar]


