Abstract
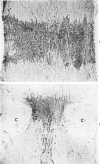
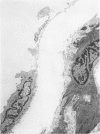
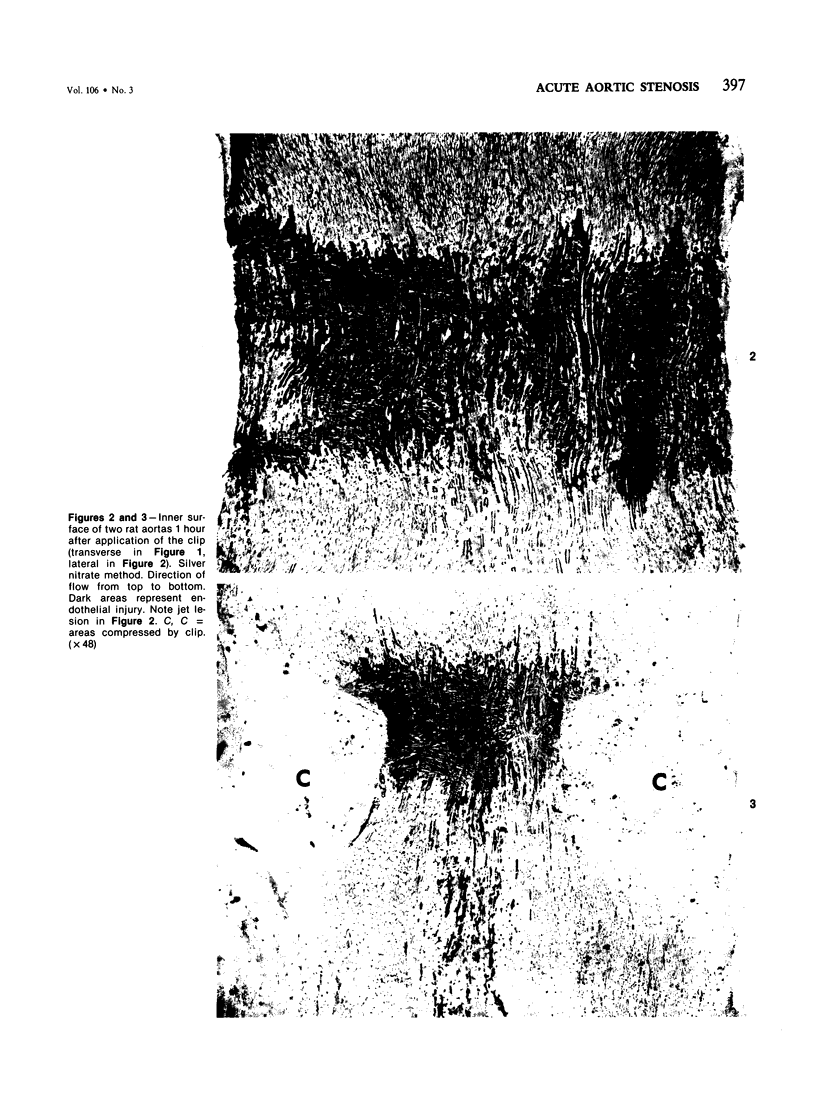
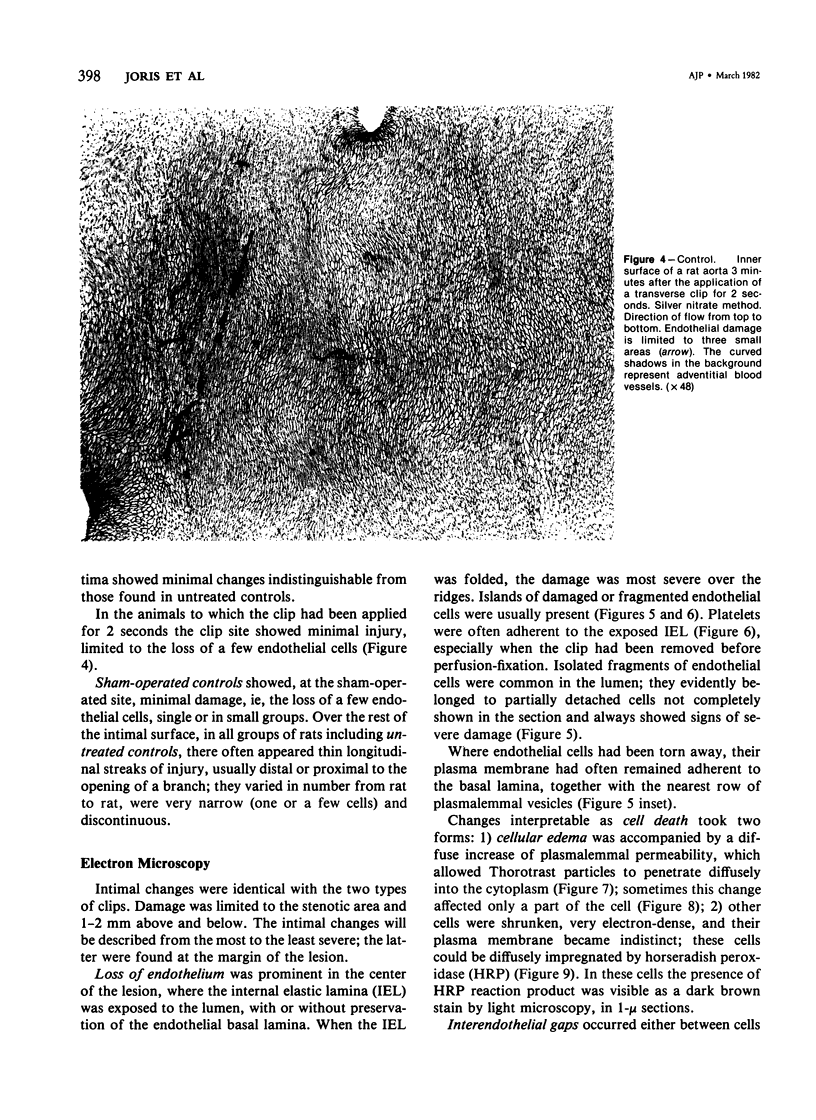
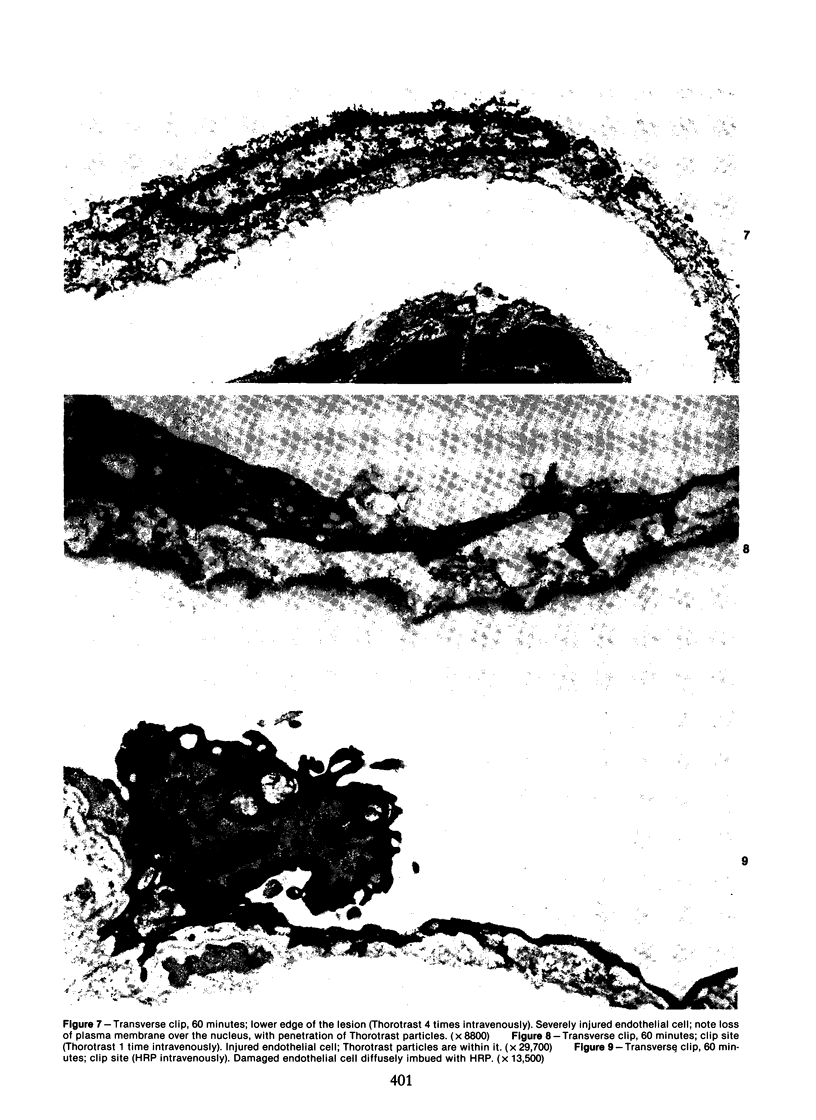
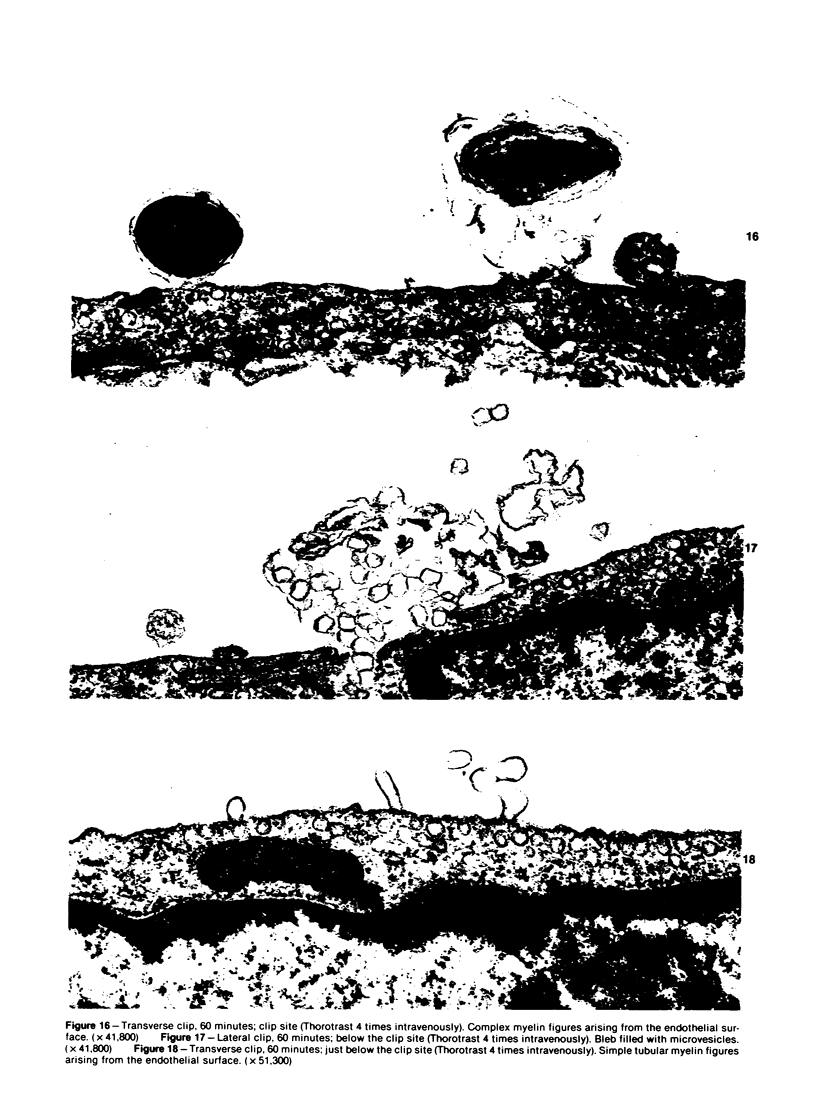
The acute effects of increased shear stress on the endothelium were studied by reducing the lumen of the rat aorta to 20-25% of normal by means of metal clips. Intimal damage in the stenotic area was assessed by light microscopy after perfusion with AgNo3 and study of the endothelium en face. Most of the endothelium was lost within 3 minutes; the extent of the damage was not increased after 1 hour. Electron-microscopic examination showed that some endothelial cells became permeable to tracers (thorium dioxide and horseradish peroxidase); platelets adhered to the exposed internal elastic membrane. Focal endothelial changes were represented by myelin figures of various kinds arising from the luminal surface and by "cellular ulcers," superficial erosions of the endothelial cells accompanied by localized cytoplasmic changes. These "ulcers" occurred more frequently over the nucleus and near junctions; they have not been described in other forms of arterial injury.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Azuma T., Fukushima T. Disturbance of blood flow as a factor of thrombus formation. Thromb Res. 1976 May;8(2 Suppl):375–380. doi: 10.1016/0049-3848(76)90079-7. [DOI] [PubMed] [Google Scholar]
- Baker F. B., Gurland J. An extension of item analysis procedures to the case of polychotomous response. Psychometrika. 1968 Sep;33(3):259–266. doi: 10.1007/BF02289326. [DOI] [PubMed] [Google Scholar]
- Baumann F. G., Imparato A. M., Kim G. E. The evolution of early fibromuscular lesions hemodynamically induced in the dog renal artery. I. Light and transmission electron microscopy. Circ Res. 1976 Dec;39(6):809–827. doi: 10.1161/01.res.39.6.809. [DOI] [PubMed] [Google Scholar]
- Camilleri J. P., Joseph D., Amat D., Fabiani J. N. Impaired sarcolemmal membrane permeability in reperfused ischemic myocardium. Ultrastructural tracer study. Virchows Arch A Pathol Anat Histol. 1980;388(1):69–76. doi: 10.1007/BF00430677. [DOI] [PubMed] [Google Scholar]
- Caro C. G., Fitz-Gerald J. M., Schroter R. C. Atheroma and arterial wall shear. Observation, correlation and proposal of a shear dependent mass transfer mechanism for atherogenesis. Proc R Soc Lond B Biol Sci. 1971 Feb 16;177(1046):109–159. doi: 10.1098/rspb.1971.0019. [DOI] [PubMed] [Google Scholar]
- Cornell R., Walker W. A., Isselbacher K. J. Small intestinal absorption of horseradish peroxidase. A cytochemical study. Lab Invest. 1971 Jul;25(1):42–48. [PubMed] [Google Scholar]
- Fallon J. T., Stehbens W. E. Venous endothelium of experimental arteriovenous fistulas in rabbits. Circ Res. 1972 Oct;31(4):546–556. doi: 10.1161/01.res.31.4.546. [DOI] [PubMed] [Google Scholar]
- Flaherty J. T., Pierce J. E., Ferrans V. J., Patel D. J., Tucker W. K., Fry D. L. Endothelial nuclear patterns in the canine arterial tree with particular reference to hemodynamic events. Circ Res. 1972 Jan;30(1):23–33. doi: 10.1161/01.res.30.1.23. [DOI] [PubMed] [Google Scholar]
- Fry D. L. Acute vascular endothelial changes associated with increased blood velocity gradients. Circ Res. 1968 Feb;22(2):165–197. doi: 10.1161/01.res.22.2.165. [DOI] [PubMed] [Google Scholar]
- Fry D. L. Certain histological and chemical responses of the vascular interface to acutely induced mechanical stress in the aorta of the dog. Circ Res. 1969 Jan;24(1):93–108. doi: 10.1161/01.res.24.1.93. [DOI] [PubMed] [Google Scholar]
- Gerrity R. G., Naito H. K. Alteration of endothelial cell surface morphology after experimental aortic coarctation. Artery. 1980;8(3):267–274. [PubMed] [Google Scholar]
- Gertz S. D., Merin G., Pasternak R. C., Gotsman M. S., Blaumanis O. R., Nelson E. Endothelial cell damage and thrombosis following partial coronary arterial constriction: relevance to the pathogenesis of myocardial infarction. Isr J Med Sci. 1978 Mar;14(3):384–388. [PubMed] [Google Scholar]
- Geyer G., Schmidt H. P., Biedermann M. Horseradish peroxidase as a label of injured cells. Histochem J. 1979 May;11(3):337–344. doi: 10.1007/BF01005032. [DOI] [PubMed] [Google Scholar]
- Glagov S. Mechanical stresses on vessels and the non-uniform distribution of atherosclerosis. Med Clin North Am. 1973 Jan;57(1):63–77. doi: 10.1016/s0025-7125(16)32302-1. [DOI] [PubMed] [Google Scholar]
- Glagov S., Ts'ao C. H. Restitution of aortic wall after sustained necrotizing transmural ligation injury. Role of blood cells and artery cells. Am J Pathol. 1975 Apr;79(1):7–30. [PMC free article] [PubMed] [Google Scholar]
- Gutstein W. H., Farrell G. A., Armellini C. Blood flow disturbance and endothelial cell injury in preatherosclerotic swine. Lab Invest. 1973 Aug;29(2):134–149. [PubMed] [Google Scholar]
- Joris I., Majno G. Cell-to-cell herniae in the arterial wall. I. The pathogenesis of vacuoles in the normal media. Am J Pathol. 1977 May;87(2):375–398. [PMC free article] [PubMed] [Google Scholar]
- Joris I., Majno G. Endothelial changes induced by arterial spasm. Am J Pathol. 1981 Mar;102(3):346–358. [PMC free article] [PubMed] [Google Scholar]
- Owen R. L. Sequential uptake of horseradish peroxidase by lymphoid follicle epithelium of Peyer's patches in the normal unobstructed mouse intestine: an ultrastructural study. Gastroenterology. 1977 Mar;72(3):440–451. [PubMed] [Google Scholar]
- Reidy M. A., Bowyer D. E. Scanning electron microscopy of arteries. The morphology of aortic endothelium in haemodynamically stressed areas associated with branches. Atherosclerosis. 1977 Feb;26(2):181–194. doi: 10.1016/0021-9150(77)90101-0. [DOI] [PubMed] [Google Scholar]
- Reidy M. A., Langille B. L. The effect of local blood flow patterns on endothelial cell morphology. Exp Mol Pathol. 1980 Jun;32(3):276–289. doi: 10.1016/0014-4800(80)90061-1. [DOI] [PubMed] [Google Scholar]
- SCHWARTZ C. J., MITCHELL J. R. Observations on localization of arterial plaques. Circ Res. 1962 Jul;11:63–73. [PubMed] [Google Scholar]
- Stehbens W. E. Haemodynamic production of lipid deposition, intimal teats, mural dissection and thrombosis in the blood vessel wall. Proc R Soc Lond B Biol Sci. 1974 Feb 12;185(1080):357–373. doi: 10.1098/rspb.1974.0024. [DOI] [PubMed] [Google Scholar]
- Stehbens W. E. The ultrastructure of the anastomosed vein of experimental arteriovenous fistulae in sheep. Am J Pathol. 1974 Aug;76(2):377–400. [PMC free article] [PubMed] [Google Scholar]
- Stetz E. M., Majno G., Joris I. Cellular pathology of the rat aorta. Pseudo-vacuoles and myo-endothelial herniae. Virchows Arch A Pathol Anat Histol. 1979 Jul 31;383(2):135–148. doi: 10.1007/BF01200895. [DOI] [PubMed] [Google Scholar]
- Svendsen E., Jørgensen L. Focal "spontaneous" alterations and loss of endothelial cells in rabbit aorta. Acta Pathol Microbiol Scand A. 1978 Jan;86(1):1–13. doi: 10.1111/j.1699-0463.1978.tb02005.x. [DOI] [PubMed] [Google Scholar]
- Ts'ao C. H. Graded endothelial injury of the rabbit aorta. With special reference to platelet deposition. Arch Pathol. 1970 Sep;90(3):222–229. [PubMed] [Google Scholar]