Abstract
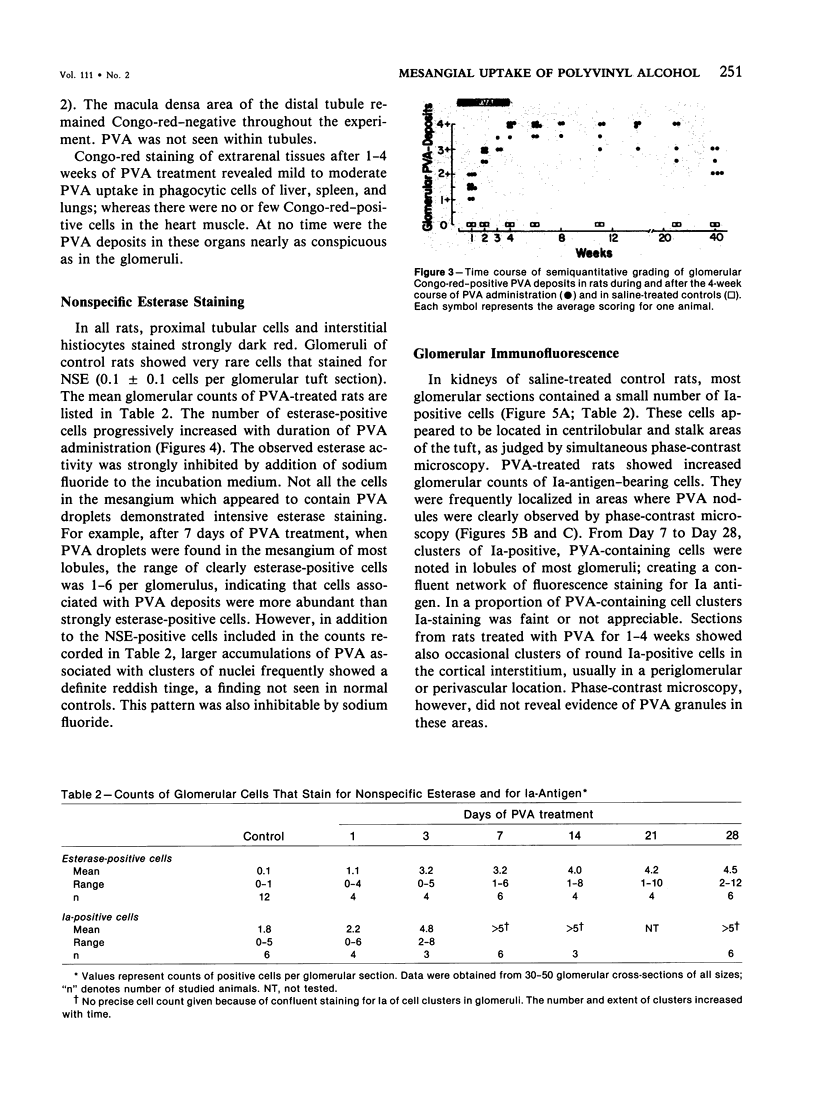
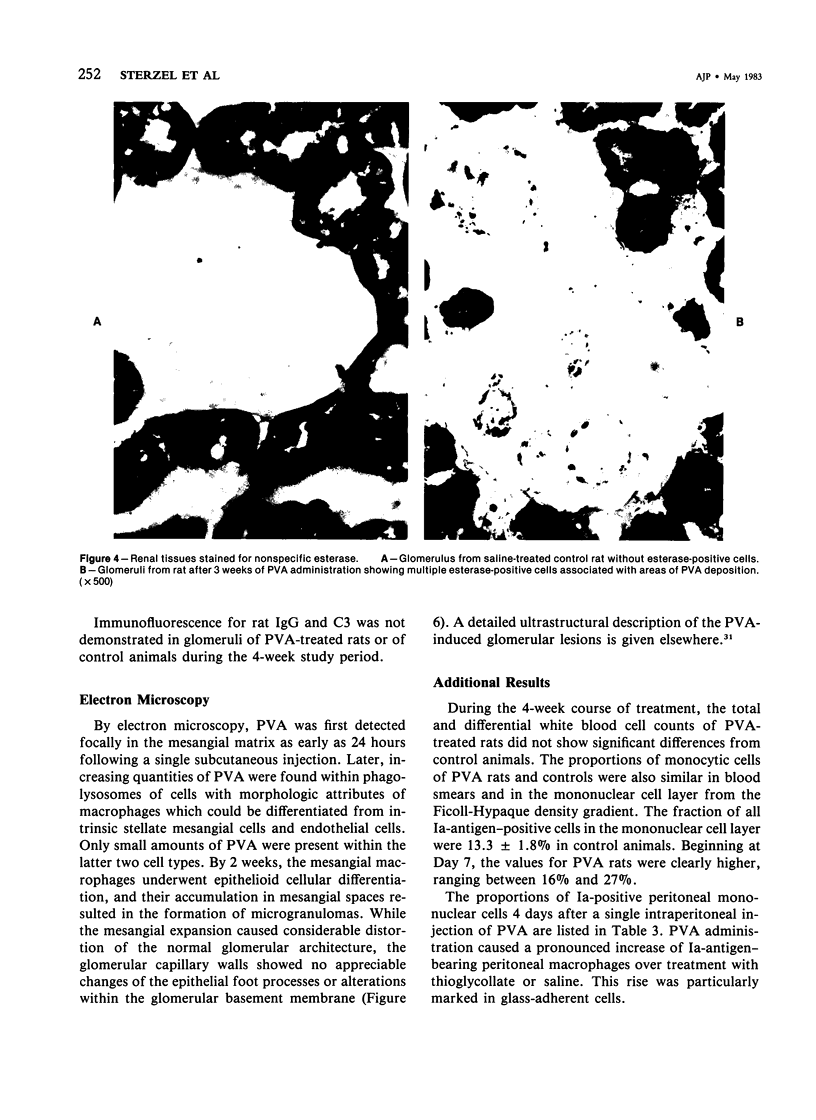
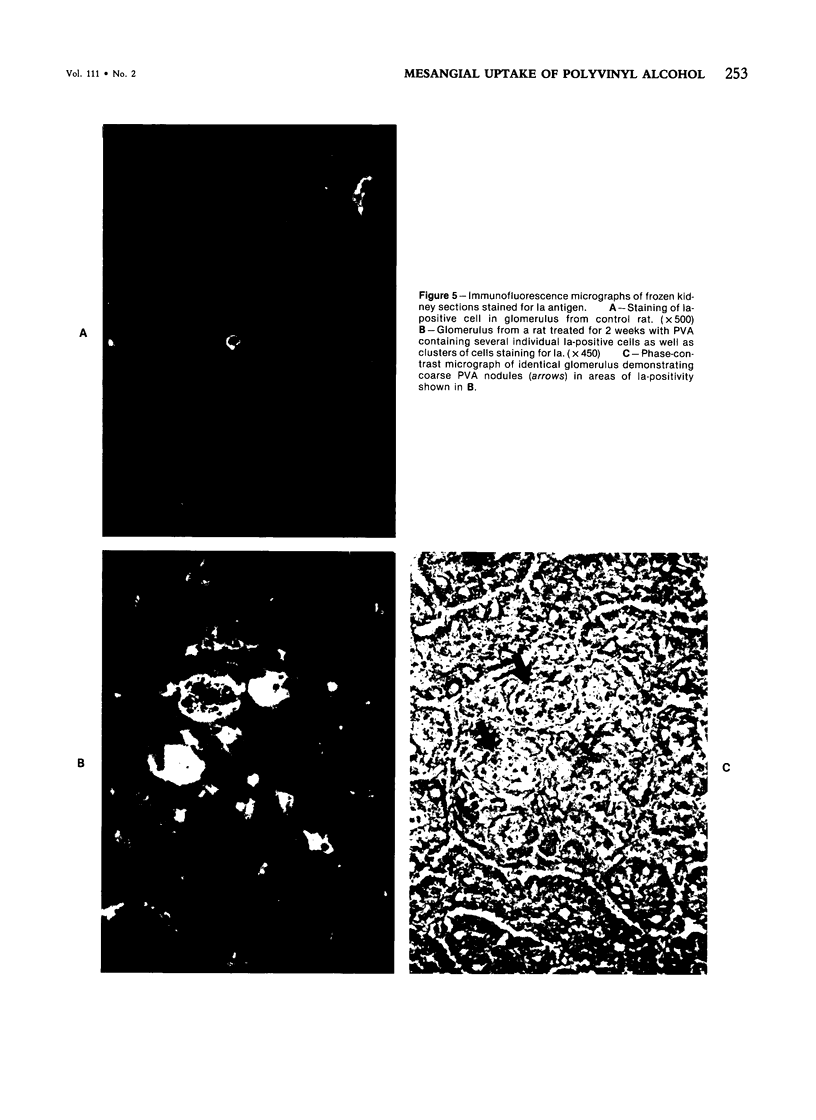
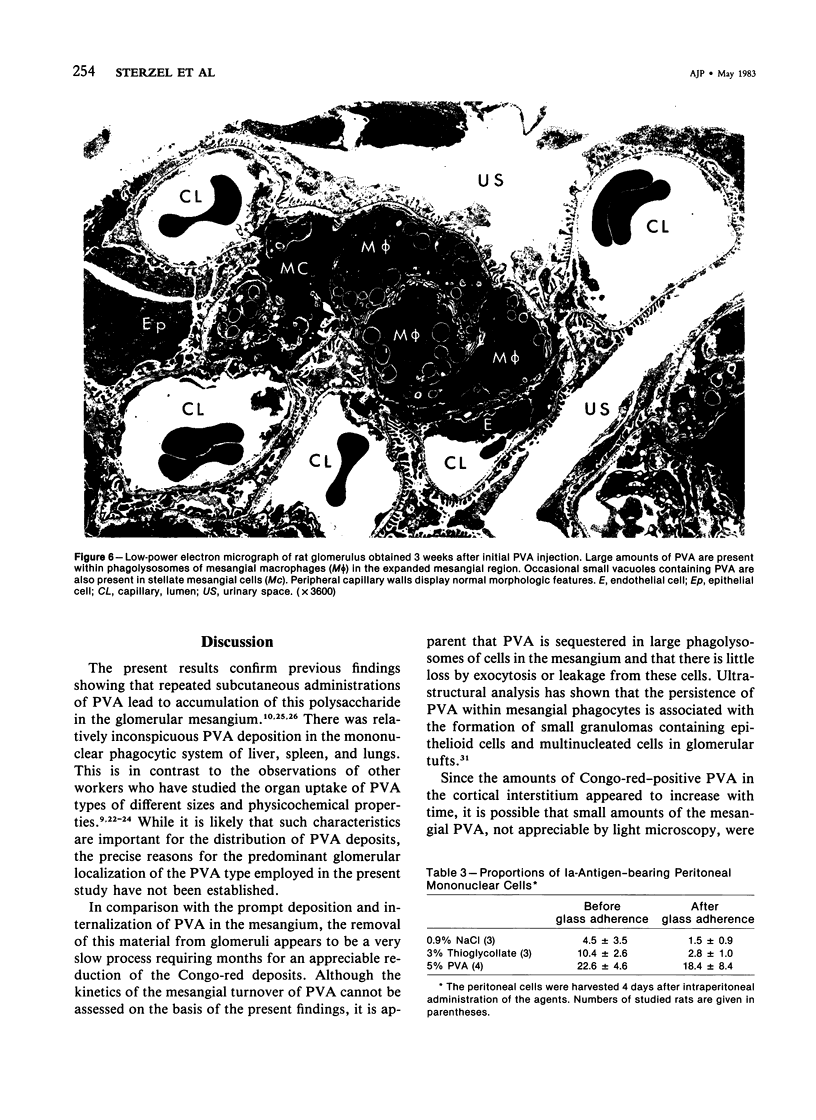
Rats received daily subcutaneous injections of the synthetic polysaccharide polyvinyl alcohol (PVA) for 1-28 days. The amount of PVA localized in the glomerular mesangium increased progressively during this time. By 28 days, all glomeruli showed extensive intracellular mesangial sequestrations of PVA, causing marked widening of mesangial areas, while the peripheral capillary loops were unaltered. Overall glomerular hypercellularity was mild to moderate, occurring mainly in areas of PVA deposition. Follow-up studies after 6, 12, 26, and 40 weeks revealed partial reduction of glomerular PVA masses. The PVA deposits were frequently associated with nonspecific esterase (NSE)-positive cells. The number of NSE-positive cells per glomerular tuft section increased from 0.1 in controls, to 2.1, 4.0, and 4.5 after 3, 14, and 28 days of PVA treatment, respectively. Similarly, glomerular counts for Ia-antigen-bearing cells rose from 2.1 in controls to 4.8 on Day 3 and showed further increases at later time periods with confluent staining of clusters of Ia-positive cells. In glomeruli, Ia-bearing cells were mainly noted in PVA-positive mesangial areas. These results indicate that PVA is taken up in the glomerulus primarily by cells that are NSE- and Ia-antigen-positive, suggesting that these cells are activated blood-borne monocyte-macrophages that sequester this polysaccharide. Clearance studies revealed that the glomerular filtration rate and effective renal plasma flow remained normal after 4 weeks of PVA injections. PVA-treated rats showed only mild elevations of urinary protein excretion. These findings indicate that confinement of marked structural and cellular alterations to the mesangium, even including the presence of infiltrating monocyte-macrophages, is compatible with absent or minimal dysfunction of the glomerular ultrafilter.
Full text
PDF

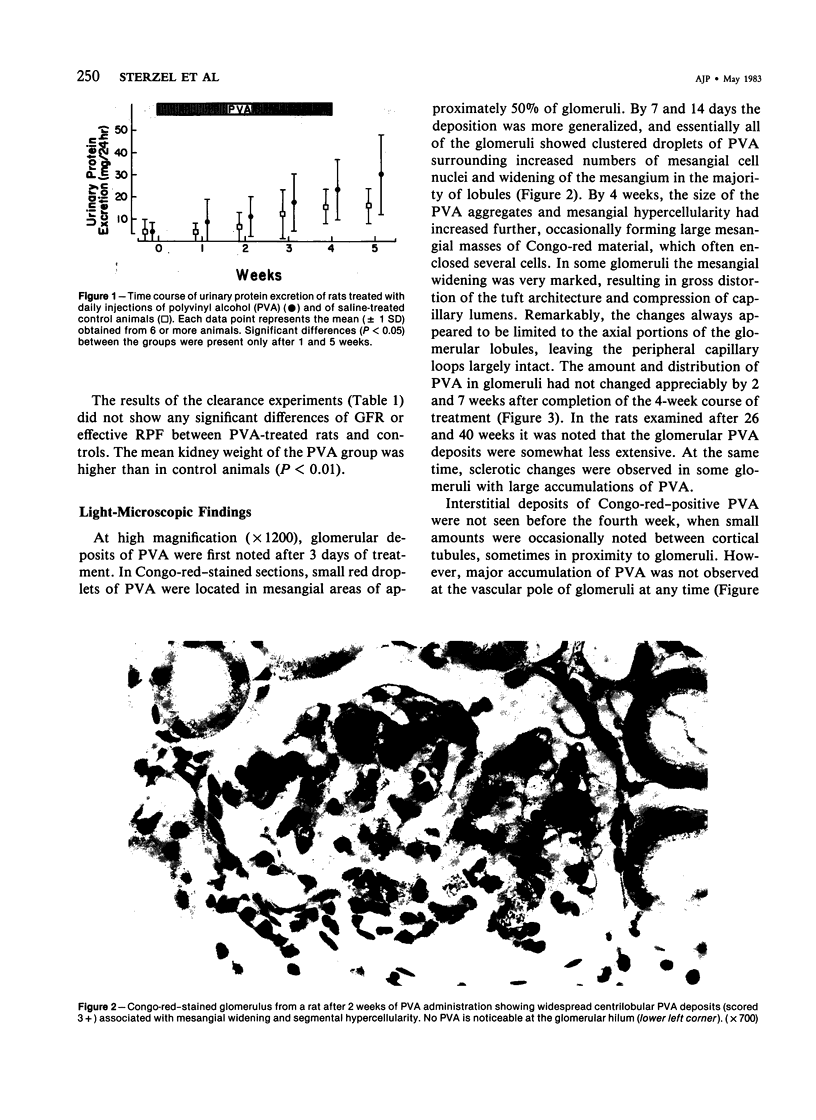



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Burkholder P. M. Functions and pathophysiology of the glomerular mesangium. Lab Invest. 1982 Feb;46(2):239–241. [PubMed] [Google Scholar]
- Calamai E. G., Beller D. I., Unanue E. R. Regulation of macrophage populations. IV. Modulation of Ia expression in bone marrow-derived macrophages. J Immunol. 1982 Apr;128(4):1692–1694. [PubMed] [Google Scholar]
- Cotran R. S. Monocytes, proliferation, and glomerulonephritis. J Lab Clin Med. 1978 Dec;92(6):837–840. [PubMed] [Google Scholar]
- Eisenbach G. M., Liew J. B., Boylan J. W., Manz N., Muir P. Effect of angiotensin on the filtration of protein in the rat kidney: a micropuncture study. Kidney Int. 1975 Aug;8(2):80–87. doi: 10.1038/ki.1975.83. [DOI] [PubMed] [Google Scholar]
- Elema J. D., Hoyer J. R., Vernier R. L. The glomerular mesangium: uptake and transport of intravenously injected colloidal carbon in rats. Kidney Int. 1976 May;9(5):395–406. doi: 10.1038/ki.1976.49. [DOI] [PubMed] [Google Scholar]
- HALL C. E., HALL O. Macromolecular hypertension and associated pathologic changes resulting from treatment with polyvinyl alcohol. Am J Pathol. 1962 Aug;41:247–257. [PMC free article] [PubMed] [Google Scholar]
- HALL C. E., HALL O. Polyvinyl alcohol: relationship of physicochemical properties to hypertension and other pathophysiologic sequelae. Lab Invest. 1963 Jul;12:721–736. [PubMed] [Google Scholar]
- Hart D. N., Fabre J. W. Demonstration and characterization of Ia-positive dendritic cells in the interstitial connective tissues of rat heart and other tissues, but not brain. J Exp Med. 1981 Aug 1;154(2):347–361. doi: 10.1084/jem.154.2.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holdsworth S. R., Neale T. J., Wilson C. B. Abrogation of macrophage-dependent injury in experimental glomerulonephritis in the rabbit. Use of an antimacrophage serum. J Clin Invest. 1981 Sep;68(3):686–698. doi: 10.1172/JCI110304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphrey J. H., Grennan D. Different macrophage populations distinguished by means of fluorescent polysaccharides. Recognition and properties of marginal-zone macrophages. Eur J Immunol. 1981 Mar;11(3):221–228. doi: 10.1002/eji.1830110311. [DOI] [PubMed] [Google Scholar]
- Klinkert W. E., LaBadie J. H., Bowers W. E. Accessory and stimulating properties of dendritic cells and macrophages isolated from various rat tissues. J Exp Med. 1982 Jul 1;156(1):1–19. doi: 10.1084/jem.156.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LATTA H., MAUNSBACH A. B., MADDEN S. C. The centrolobular region of the renal glomerulus studied by electron microscopy. J Ultrastruct Res. 1960 Dec;4:455–472. doi: 10.1016/s0022-5320(60)80033-0. [DOI] [PubMed] [Google Scholar]
- Lee S., Vernier R. L. Immunoelectron microscopy of the glomerular mesangial uptake and transport of aggregated human albumin in the mouse. Lab Invest. 1980 Jan;42(1):44–58. [PubMed] [Google Scholar]
- Leiper J. M., Thomson D., MacDonald M. K. Uptake and transport of Imposil by the glomerular mesangium in the mouse. Lab Invest. 1977 Nov;37(5):526–533. [PubMed] [Google Scholar]
- Magil A. B., Wadsworth L. D., Loewen M. Monocytes and human renal glomerular disease: a quantitative evaluation. Lab Invest. 1981 Jan;44(1):27–33. [PubMed] [Google Scholar]
- Mancilla-Jimenez R., Bellon B., Kuhn J., Belair M. F., Rouchon M., Druet P., Bariety J. Phagocytosis of heat-aggregated immunoglobulins by mesangial cells: an immunoperoxidase and acid phosphatase study. Lab Invest. 1982 Mar;46(3):243–253. [PubMed] [Google Scholar]
- Mauer S. M., Numata M., Sutherland D. E. The effects of polyvinyl alcohol on the uptake and processing of colloidal carbon by the glomerular mesangium in rats. Lab Invest. 1979 Dec;41(6):475–482. [PubMed] [Google Scholar]
- McMaster W. R., Williams A. F. Identification of Ia glycoproteins in rat thymus and purification from rat spleen. Eur J Immunol. 1979 Jun;9(6):426–433. doi: 10.1002/eji.1830090603. [DOI] [PubMed] [Google Scholar]
- Michael A. F., Keane W. F., Raij L., Vernier R. L., Mauer S. M. The glomerular mesangium. Kidney Int. 1980 Feb;17(2):141–154. doi: 10.1038/ki.1980.18. [DOI] [PubMed] [Google Scholar]
- Monga G., Mazzucco G., di Belgiojoso G. B., Busnach G. Monocyte infiltration and glomerular hypercellularity in human acute and persistent glomerulonephritis. Light and electron microscopic, immunofluorescence, and histochemical investigation on twenty-eight cases. Lab Invest. 1981 Apr;44(4):381–387. [PubMed] [Google Scholar]
- Pardo V., Shapiro A. P. Ultrastructural glomerular lesions produced by synthetic polysaccharides. Lab Invest. 1966 Mar;15(3):617–628. [PubMed] [Google Scholar]
- Schreiner G. F., Cotran R. S., Pardo V., Unanue E. R. A mononuclear cell component in experimental immunological glomerulonephritis. J Exp Med. 1978 Feb 1;147(2):369–384. doi: 10.1084/jem.147.2.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiner G. F., Kiely J. M., Cotran R. S., Unanue E. R. Characterization of resident glomerular cells in the rat expressing Ia determinants and manifesting genetically restricted interactions with lymphocytes. J Clin Invest. 1981 Oct;68(4):920–931. doi: 10.1172/JCI110347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinman R. M., Kaplan G., Witmer M. D., Cohn Z. A. Identification of a novel cell type in peripheral lymphoid organs of mice. V. Purification of spleen dendritic cells, new surface markers, and maintenance in vitro. J Exp Med. 1979 Jan 1;149(1):1–16. doi: 10.1084/jem.149.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sterzel R. B., Ehrich J. H., Lucia H., Thomson D., Kashgarian M. Mesangial disposal of glomerular immune deposits in acute malarial glomerulonephritis of rats. Lab Invest. 1982 Feb;46(2):209–214. [PubMed] [Google Scholar]
- Sterzel R. B., Pabst R. The temporal relationship between glomerular cell proliferation and monocyte infiltration in experimental glomerulonephritis. Virchows Arch B Cell Pathol Incl Mol Pathol. 1982;38(3):337–350. doi: 10.1007/BF02892829. [DOI] [PubMed] [Google Scholar]
- Striker G. E., Mannik M., Tung M. Y. Role of marrow-derived monocytes and mesangial cells in removal of immune complexes from renal glomeruli. J Exp Med. 1979 Jan 1;149(1):127–136. doi: 10.1084/jem.149.1.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Voorhis W. C., Hair L. S., Steinman R. M., Kaplan G. Human dendritic cells. Enrichment and characterization from peripheral blood. J Exp Med. 1982 Apr 1;155(4):1172–1187. doi: 10.1084/jem.155.4.1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yam L. T., Li C. Y., Crosby W. H. Cytochemical identification of monocytes and granulocytes. Am J Clin Pathol. 1971 Mar;55(3):283–290. doi: 10.1093/ajcp/55.3.283. [DOI] [PubMed] [Google Scholar]