Abstract
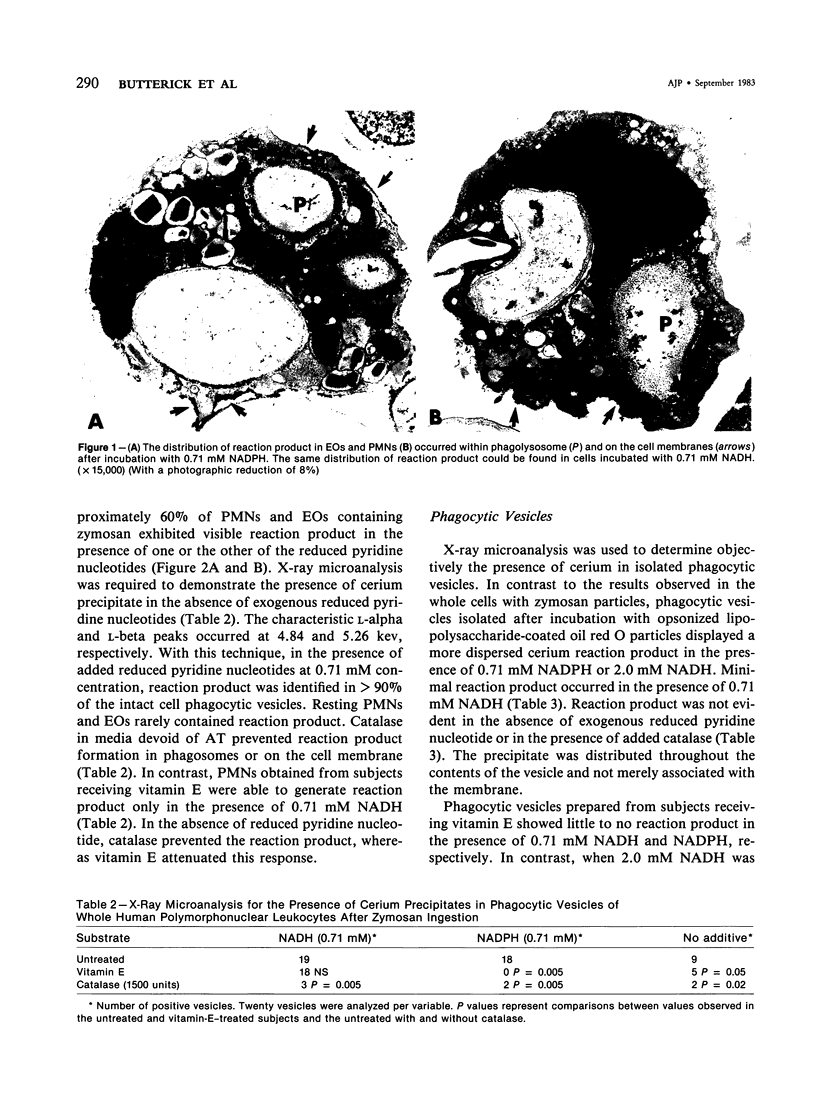
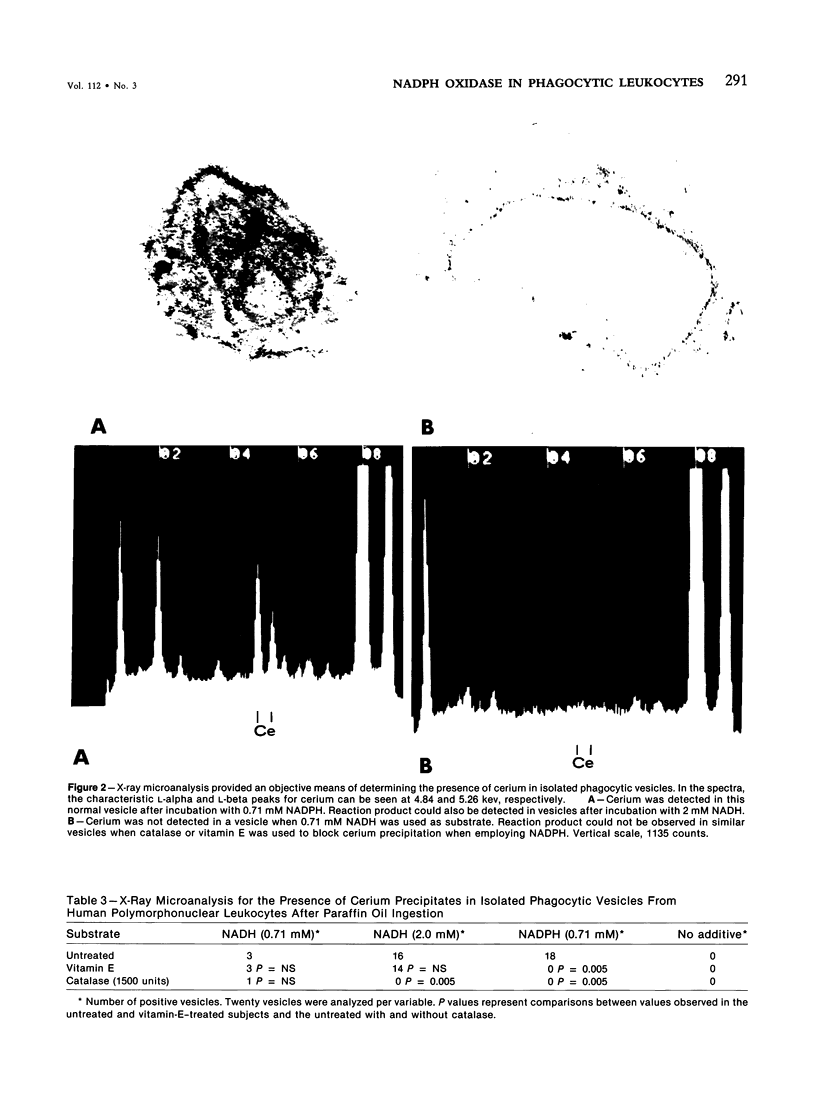
The cellular sites of H2O2 formation in phagocytizing granulocytes have been identified with cerium chloride. A precipitate was visible in phagosomes and on plasma membranes from intact normal cells in the presence of either 0.71 mM NADH or NADPH. X-ray microanalysis permitted identification of cerium deposition within the phagosomes even in the absence of reduced pyridine nucleotides. Catalase ablated the formation of the reaction product. Intact granulocytes obtained from subjects receiving 1600 units of vitamin E daily for 2 weeks exhibited reaction product in the presence of NADH but not NADPH. Intact cells from subjects treated with vitamin E demonstrated diminished numbers of phagocytic vesicles containing reaction product. During phagocytosis the granulocytes treated with vitamin E consumed oxygen but exhibited significantly reduced rates of hydrogen-peroxide-dependent glucose-1-14C oxidation to 14CO2. Isolated phagocytic vesicles obtained from granulocytes after ingestion of opsonized lipopolysaccharide-paraffin oil droplets contained reaction product when exposed to 0.71 mM NADPH. No reaction product was evident at 0.71 mM NADH but was evident at 2.0 mM NADH. Isolated phagocytic vesicles from the granulocytes of subjects receiving vitamin E exhibited reaction product only in the presence of NADH. These observations suggest that vitamin E interferes with the electron transport chain apparently required for the oxidation of NADPH to form H2O2 in the phagocytizing granulocyte.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Babior B. M., Kipnes R. S. Superoxide-forming enzyme from human neutrophils: evidence for a flavin requirement. Blood. 1977 Sep;50(3):517–524. [PubMed] [Google Scholar]
- Babior B. M. Oxygen-dependent microbial killing by phagocytes (second of two parts). N Engl J Med. 1978 Mar 30;298(13):721–725. doi: 10.1056/NEJM197803302981305. [DOI] [PubMed] [Google Scholar]
- Baehner R. L., Boxer L. A., Allen J. M., Davis J. Autooxidation as a basis for altered function by polymorphonuclear leukocytes. Blood. 1977 Aug;50(2):327–335. [PubMed] [Google Scholar]
- Baehner R. L., Johnston R. B., Jr Metabolic and bactericidal activities of human eosinophils. Br J Haematol. 1971 Mar;20(3):277–285. doi: 10.1111/j.1365-2141.1971.tb07038.x. [DOI] [PubMed] [Google Scholar]
- Baehner R. L., Nathan D. G., Karnovsky M. L. Correction of metabolic deficiencies in the leukocytes of patients with chronic granulomatous disease. J Clin Invest. 1970 May;49(5):865–870. doi: 10.1172/JCI106305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boxer L. A., Stossel T. P. Effects of anti-human neutrophil antibodies in vitro. Quantitative studies. J Clin Invest. 1974 Jun;53(6):1534–1545. doi: 10.1172/JCI107704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briggs R. T., Drath D. B., Karnovsky M. L., Karnovsky M. J. Localization of NADH oxidase on the surface of human polymorphonuclear leukocytes by a new cytochemical method. J Cell Biol. 1975 Dec;67(3):566–586. doi: 10.1083/jcb.67.3.566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen H. J., Newburger P. E., Chovaniec M. E. NAD(P)H-dependent superoxide production by phagocytic vesicles from guinea pig and human granulocytes. J Biol Chem. 1980 Jul 25;255(14):6584–6588. [PubMed] [Google Scholar]
- Crawford D. R., Schneider D. L. Identification of ubiquinone-50 in human neutrophils and its role in microbicidal events. J Biol Chem. 1982 Jun 25;257(12):6662–6668. [PubMed] [Google Scholar]
- GOODHUE C. T., RISLEY H. A. REACTIONS OF VITAMIN E WITH PEROXIDES. II. REACTION OF BENZOYL PEROXIDE WITH D-ALPHA-TOCOPHEROL IN ALCOHOLS. Biochemistry. 1965 May;4:854–858. doi: 10.1021/bi00881a009. [DOI] [PubMed] [Google Scholar]
- Green T. R., Schaefer R. E. Intrinsic dichlorophenolindophenol reductase activity associated with the superoxide-generating oxidoreductase of human granulocytes. Biochemistry. 1981 Dec 22;20(26):7483–7487. doi: 10.1021/bi00529a024. [DOI] [PubMed] [Google Scholar]
- Ohno Y., Hirai K., Kanoh T., Uchino H., Ogawa K. Subcellular localization of H2O2 production in human neutrophils stimulated with particles and an effect of cytochalasin-B on the cells. Blood. 1982 Jul;60(1):253–260. [PubMed] [Google Scholar]
- Root R. K., Metcalf J., Oshino N., Chance B. H2O2 release from human granulocytes during phagocytosis. I. Documentation, quantitation, and some regulating factors. J Clin Invest. 1975 May;55(5):945–955. doi: 10.1172/JCI108024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segal A. W., Jones O. T. Reduction and subsequent oxidation of a cytochrome b of human neutrophils after stimulation with phorbol myristate acetate. Biochem Biophys Res Commun. 1979 May 14;88(1):130–134. doi: 10.1016/0006-291x(79)91706-6. [DOI] [PubMed] [Google Scholar]
- Spurr A. R. A low-viscosity epoxy resin embedding medium for electron microscopy. J Ultrastruct Res. 1969 Jan;26(1):31–43. doi: 10.1016/s0022-5320(69)90033-1. [DOI] [PubMed] [Google Scholar]
- Stossel T. P., Pollard T. D., Mason R. J., Vaughan M. Isolation and properties of phagocytic vesicles from polymorphonuclear leukocytes. J Clin Invest. 1971 Aug;50(8):1745–1747. doi: 10.1172/JCI106664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winterbourn C. C. Cytochrome c reduction by semiquinone radicals can be indirectly inhibited by superoxide dismutase. Arch Biochem Biophys. 1981 Jun;209(1):159–167. doi: 10.1016/0003-9861(81)90268-x. [DOI] [PubMed] [Google Scholar]