Abstract
1. We have investigated the modulation of N-methyl-D-aspartate (NMDA) receptor activation by the sulphydryl redox reagents dithiothreitol (DTT) and 5,5-dithio-bis-2-nitrobenzoic acid (DTNB). 2. Increases in [3H]-MK-801 binding produced by glutamate, glycine and spermidine were enhanced by DTT (2mM) and diminished by DTNB (0.5 mM). 3. The inhibition of [3H]-MK-801 binding by CGS 19755 and 7-chlorokynurenate was not altered by 2 mM DTT. However, the potency of the competitive polyamine antagonist, arcaine, was decreased by DTT. 4. NMDA-induced Ca2+ fluxes into primary cultures of rat forebrain neurones were enhanced by DTT in a DTNB-reversible fashion. In addition to augmenting the magnitude of NMDA-induced increase in intracellular free Ca2+, 10 mM DTT also prolonged the duration of the Ca2+ signal. However, DTT had no effect on the increase in Ca2+ produced by depolarizing neurones with 50 mM KCl. 5. These studies show that the reduction of disulphide bonds on the NMDA receptor complex by DTT increases activation. The precise site of these groups remains unclear but they are unlikely to form an integral part of the glutamate, glycine or polyamine binding domains. The enhancement of the activation of the NMDA receptor by DTT is associated with increased Ca2+ fluxes. The possible pathophysiological consequences of receptor reduction are discussed.
Full text
PDF

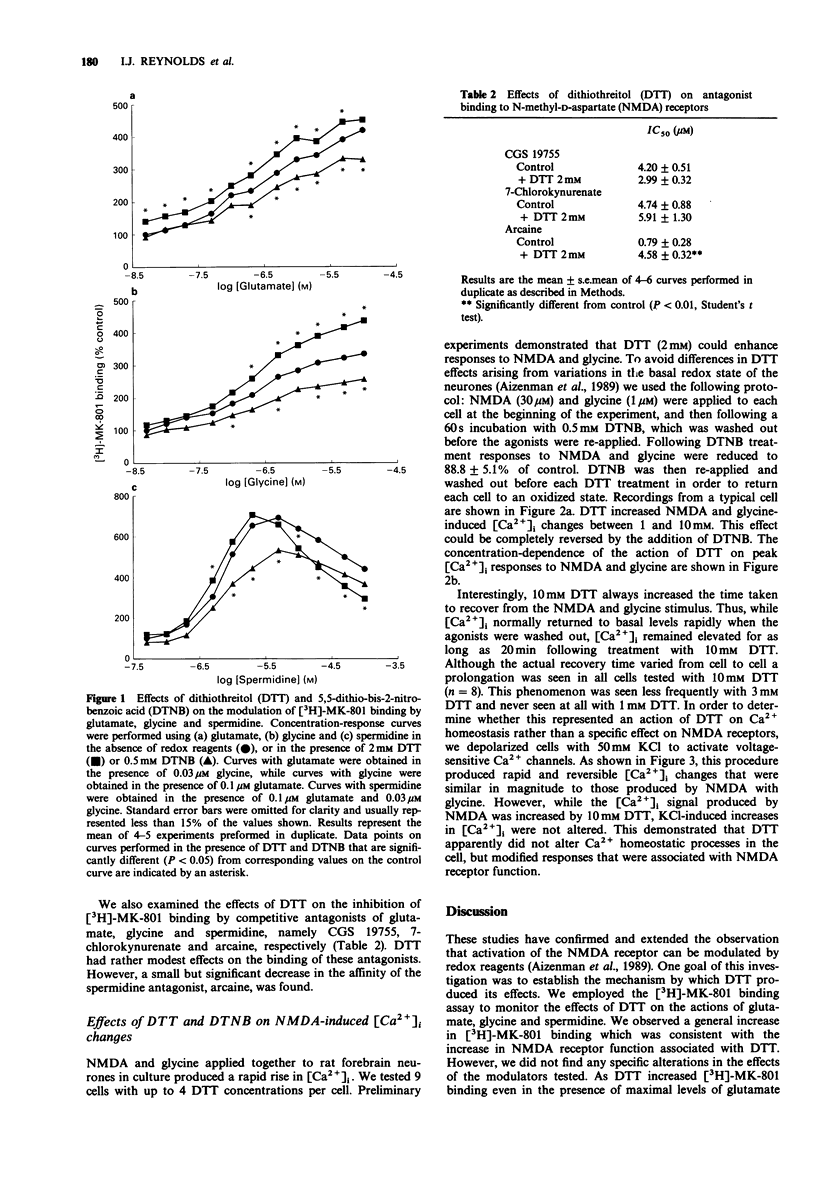
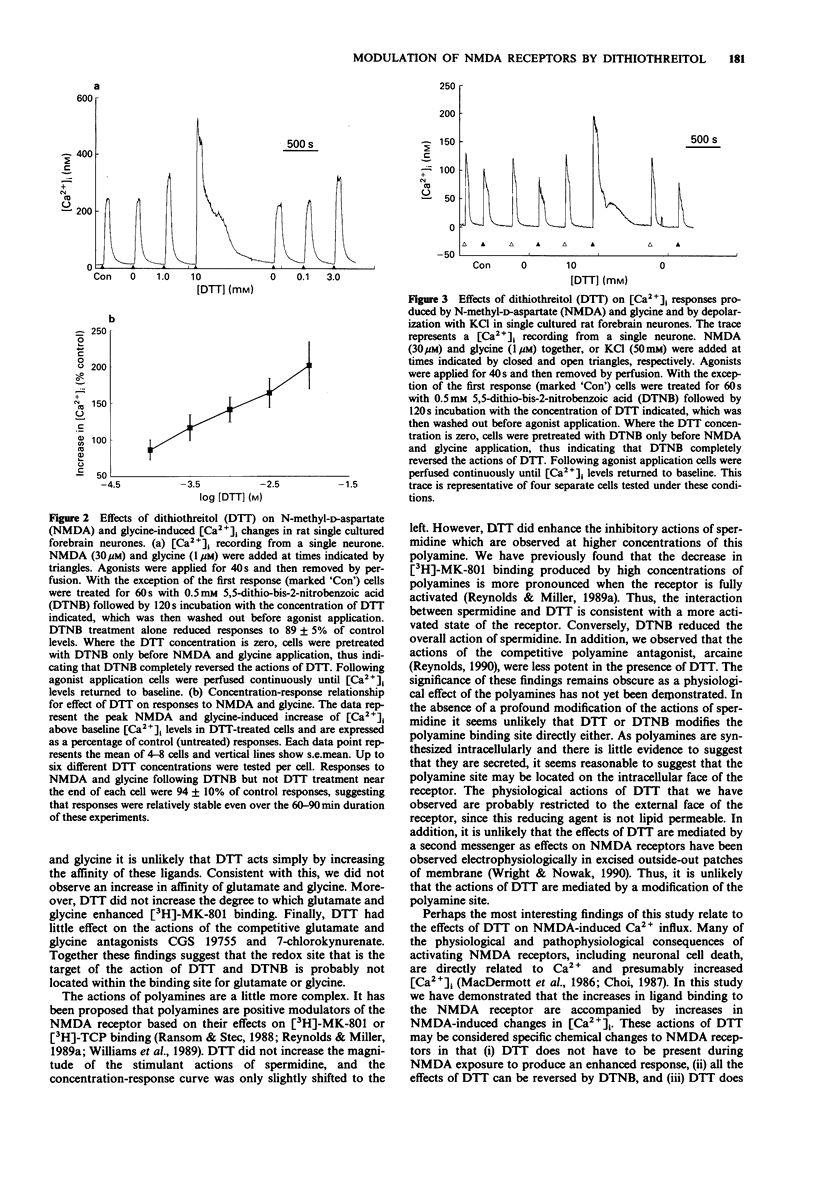

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aizenman E., Lipton S. A., Loring R. H. Selective modulation of NMDA responses by reduction and oxidation. Neuron. 1989 Mar;2(3):1257–1263. doi: 10.1016/0896-6273(89)90310-3. [DOI] [PubMed] [Google Scholar]
- Choi D. W. Ionic dependence of glutamate neurotoxicity. J Neurosci. 1987 Feb;7(2):369–379. doi: 10.1523/JNEUROSCI.07-02-00369.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collingridge G. L., Lester R. A. Excitatory amino acid receptors in the vertebrate central nervous system. Pharmacol Rev. 1989 Jun;41(2):143–210. [PubMed] [Google Scholar]
- Foster A. C., Wong E. H. The novel anticonvulsant MK-801 binds to the activated state of the N-methyl-D-aspartate receptor in rat brain. Br J Pharmacol. 1987 Jun;91(2):403–409. doi: 10.1111/j.1476-5381.1987.tb10295.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- Levy D. I., Sucher N. J., Lipton S. A. Redox modulation of NMDA receptor-mediated toxicity in mammalian central neurons. Neurosci Lett. 1990 Mar 14;110(3):291–296. doi: 10.1016/0304-3940(90)90862-4. [DOI] [PubMed] [Google Scholar]
- MacDermott A. B., Mayer M. L., Westbrook G. L., Smith S. J., Barker J. L. NMDA-receptor activation increases cytoplasmic calcium concentration in cultured spinal cord neurones. 1986 May 29-Jun 4Nature. 321(6069):519–522. doi: 10.1038/321519a0. [DOI] [PubMed] [Google Scholar]
- Murphy S. N., Thayer S. A., Miller R. J. The effects of excitatory amino acids on intracellular calcium in single mouse striatal neurons in vitro. J Neurosci. 1987 Dec;7(12):4145–4158. doi: 10.1523/JNEUROSCI.07-12-04145.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulsinelli W. A. Selective neuronal vulnerability: morphological and molecular characteristics. Prog Brain Res. 1985;63:29–37. doi: 10.1016/S0079-6123(08)61973-1. [DOI] [PubMed] [Google Scholar]
- Ransom R. W., Stec N. L. Cooperative modulation of [3H]MK-801 binding to the N-methyl-D-aspartate receptor-ion channel complex by L-glutamate, glycine, and polyamines. J Neurochem. 1988 Sep;51(3):830–836. doi: 10.1111/j.1471-4159.1988.tb01818.x. [DOI] [PubMed] [Google Scholar]
- Reynolds I. J. Arcaine is a competitive antagonist of the polyamine site on the NMDA receptor. Eur J Pharmacol. 1990 Feb 27;177(3):215–216. doi: 10.1016/0014-2999(90)90274-a. [DOI] [PubMed] [Google Scholar]
- Reynolds I. J., Miller R. J. Ifenprodil is a novel type of N-methyl-D-aspartate receptor antagonist: interaction with polyamines. Mol Pharmacol. 1989 Nov;36(5):758–765. [PubMed] [Google Scholar]
- Reynolds I. J., Miller R. J. Multiple sites for the regulation of the N-methyl-D-aspartate receptor. Mol Pharmacol. 1988 Jun;33(6):581–584. [PubMed] [Google Scholar]
- Reynolds I. J., Miller R. J. Muscarinic agonists cause calcium influx and calcium mobilization in forebrain neurons in vitro. J Neurochem. 1989 Jul;53(1):226–233. doi: 10.1111/j.1471-4159.1989.tb07318.x. [DOI] [PubMed] [Google Scholar]
- Reynolds I. J., Murphy S. N., Miller R. J. 3H-labeled MK-801 binding to the excitatory amino acid receptor complex from rat brain is enhanced by glycine. Proc Natl Acad Sci U S A. 1987 Nov;84(21):7744–7748. doi: 10.1073/pnas.84.21.7744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolliver J. M., Pellmar T. C. Dithiothreitol elicits epileptiform activity in CA1 of the guinea pig hippocampal slice. Brain Res. 1987 Feb 24;404(1-2):133–141. doi: 10.1016/0006-8993(87)91364-3. [DOI] [PubMed] [Google Scholar]
- Tolliver J. M., Pellmar T. C. Effects of dithiothreitol, a sulfhydryl reducing agent, on CA1 pyramidal cells of the guinea pig hippocampus in vitro. Brain Res. 1988 Jul 19;456(1):49–56. doi: 10.1016/0006-8993(88)90345-9. [DOI] [PubMed] [Google Scholar]
- Wieloch T. Neurochemical correlates to selective neuronal vulnerability. Prog Brain Res. 1985;63:69–85. doi: 10.1016/S0079-6123(08)61976-7. [DOI] [PubMed] [Google Scholar]
- Williams K., Romano C., Molinoff P. B. Effects of polyamines on the binding of [3H]MK-801 to the N-methyl-D-aspartate receptor: pharmacological evidence for the existence of a polyamine recognition site. Mol Pharmacol. 1989 Oct;36(4):575–581. [PubMed] [Google Scholar]


