Abstract
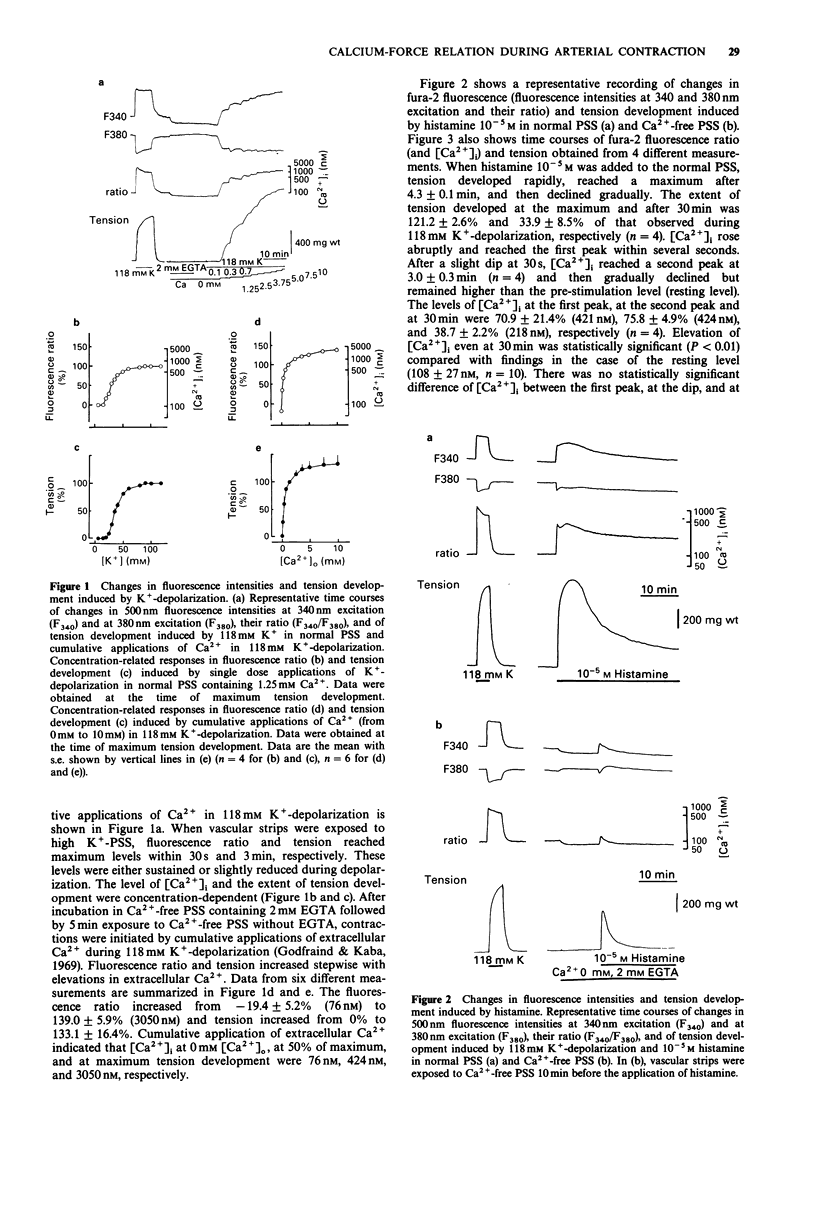
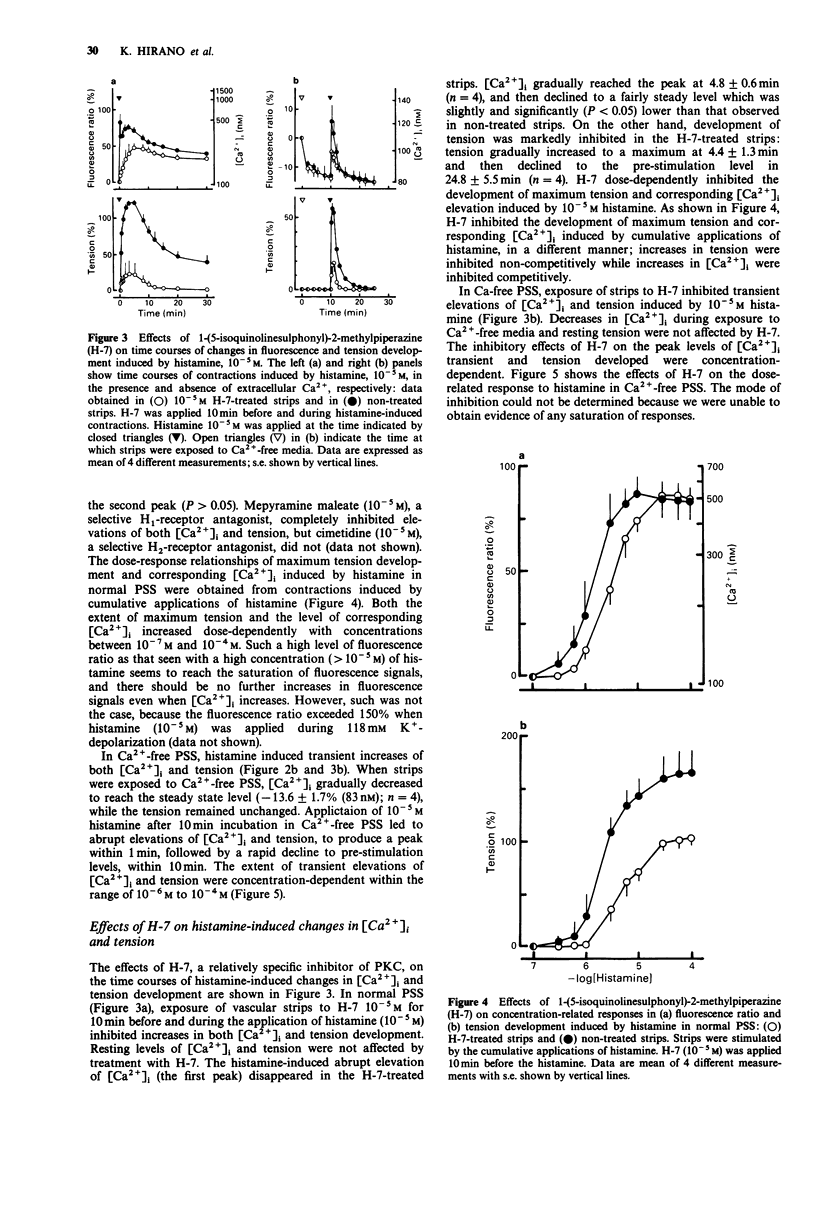
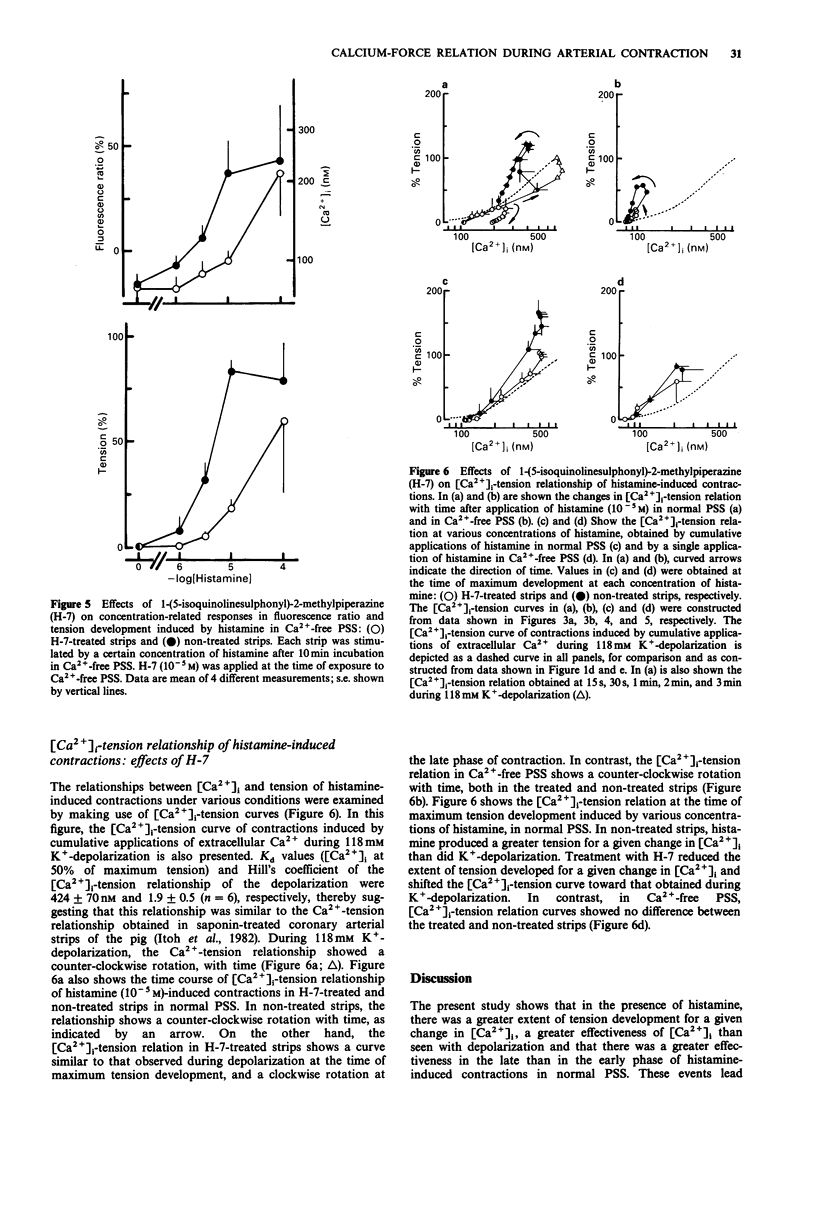
1. We examined temporal changes in the relationship between cytosolic calcium concentrations ([Ca2+]i) and developed tension during histamine-induced contractions of strips of the coronary artery of the pig, by making use of simultaneous measurements of fura-2 fluorescence and force. 2. The relationship between [Ca2+]i and developed tension observed with cumulative applications of extracellular Ca2+ ([Ca2+]o), ranging from 0 mM to 10 mM, during 118 mM K(+)-depolarization was similar to that observed in chemically skinned strips of the porcine coronary artery, as noted by other investigators. [Ca2+]i at 0 mM [Ca2+]o, at 50% of maximum, and at maximum tension development were 76 nM, 424 nM, and 3050 nM, respectively. 3. Cumulative applications of histamine induced dose-dependent increases in [Ca2+]i and tension and the extent of tension for a given change in [Ca2+]i increased, i.e. greater effectiveness of [Ca2+]i-tension relationship, than seen with K(+)-depolarization. 4. When histamine 10(-5) M was applied, [Ca2+]i abruptly rose and reached the first peak within several seconds. After a slight dip at 30 s, [Ca2+]i reached a second peak at 3 min, and then gradually declined. On the other hand, tension developed rapidly reached a maximum at 4 min, then gradually declined. The relation between [Ca2+]i and tension in the early, rising phase of contraction was similar to that obtained during depolarization. At the time of maximum tension development, the relation was greater than that observed during depolarization, which persisted in the phase of declining tension.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bengur A. R., Robinson E. A., Appella E., Sellers J. R. Sequence of the sites phosphorylated by protein kinase C in the smooth muscle myosin light chain. J Biol Chem. 1987 Jun 5;262(16):7613–7617. [PubMed] [Google Scholar]
- Bruschi G., Bruschi M. E., Regolisti G., Borghetti A. Myoplasmic Ca2+-force relationship studied with fura-2 during stimulation of rat aortic smooth muscle. Am J Physiol. 1988 May;254(5 Pt 2):H840–H854. doi: 10.1152/ajpheart.1988.254.5.H840. [DOI] [PubMed] [Google Scholar]
- Chatterjee M., Tejada M. Phorbol ester-induced contraction in chemically skinned vascular smooth muscle. Am J Physiol. 1986 Sep;251(3 Pt 1):C356–C361. doi: 10.1152/ajpcell.1986.251.3.C356. [DOI] [PubMed] [Google Scholar]
- Danthuluri N. R., Deth R. C. Phorbol ester-induced contraction of arterial smooth muscle and inhibition of alpha-adrenergic response. Biochem Biophys Res Commun. 1984 Dec 28;125(3):1103–1109. doi: 10.1016/0006-291x(84)91397-4. [DOI] [PubMed] [Google Scholar]
- Daum P. R., Downes C. P., Young J. M. Histamine stimulation of inositol 1-phosphate accumulation in lithium-treated slices from regions of guinea pig brain. J Neurochem. 1984 Jul;43(1):25–32. doi: 10.1111/j.1471-4159.1984.tb06674.x. [DOI] [PubMed] [Google Scholar]
- Donaldson J., Hill S. J. Histamine-induced hydrolysis of polyphosphoinositides in guinea-pig ileum and brain. Eur J Pharmacol. 1986 May 27;124(3):255–265. doi: 10.1016/0014-2999(86)90226-8. [DOI] [PubMed] [Google Scholar]
- Endo T., Naka M., Hidaka H. Ca2+-phospholipid dependent phosphorylation of smooth muscle myosin. Biochem Biophys Res Commun. 1982 Apr 14;105(3):942–948. doi: 10.1016/0006-291x(82)91061-0. [DOI] [PubMed] [Google Scholar]
- Godfraind T., Kaba A. Blockade or reversal of the contraction induced by calcium and adrenaline in depolarized arterial smooth muscle. Br J Pharmacol. 1969 Jul;36(3):549–560. doi: 10.1111/j.1476-5381.1969.tb08010.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griendling K. K., Rittenhouse S. E., Brock T. A., Ekstein L. S., Gimbrone M. A., Jr, Alexander R. W. Sustained diacylglycerol formation from inositol phospholipids in angiotensin II-stimulated vascular smooth muscle cells. J Biol Chem. 1986 May 5;261(13):5901–5906. [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- Hagen M., Paegelow I. Histamine H1-and H2-receptors in coronary arteries of pigs. Agents Actions. 1979 Aug;9(3):253–256. doi: 10.1007/BF01966697. [DOI] [PubMed] [Google Scholar]
- Hidaka H., Inagaki M., Kawamoto S., Sasaki Y. Isoquinolinesulfonamides, novel and potent inhibitors of cyclic nucleotide dependent protein kinase and protein kinase C. Biochemistry. 1984 Oct 9;23(21):5036–5041. doi: 10.1021/bi00316a032. [DOI] [PubMed] [Google Scholar]
- Himpens B., Somlyo A. P. Free-calcium and force transients during depolarization and pharmacomechanical coupling in guinea-pig smooth muscle. J Physiol. 1988 Jan;395:507–530. doi: 10.1113/jphysiol.1988.sp016932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikebe M., Hartshorne D. J., Elzinga M. Phosphorylation of the 20,000-dalton light chain of smooth muscle myosin by the calcium-activated, phospholipid-dependent protein kinase. Phosphorylation sites and effects of phosphorylation. J Biol Chem. 1987 Jul 15;262(20):9569–9573. [PubMed] [Google Scholar]
- Ikebe M., Inagaki M., Kanamaru K., Hidaka H. Phosphorylation of smooth muscle myosin light chain kinase by Ca2+-activated, phospholipid-dependent protein kinase. J Biol Chem. 1985 Apr 25;260(8):4547–4550. [PubMed] [Google Scholar]
- Itoh T., Kajiwara M., Kitamura K., Kuriyama H. Roles of stored calcium on the mechanical response evoked in smooth muscle cells of the porcine coronary artery. J Physiol. 1982 Jan;322:107–125. doi: 10.1113/jphysiol.1982.sp014026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh T., Kubota Y., Kuriyama H. Effects of a phorbol ester on acetylcholine-induced Ca2+ mobilization and contraction in the porcine coronary artery. J Physiol. 1988 Mar;397:401–419. doi: 10.1113/jphysiol.1988.sp017008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang M. J., Morgan K. G. Intracellular calcium levels in phorbol ester-induced contractions of vascular muscle. Am J Physiol. 1987 Dec;253(6 Pt 2):H1365–H1371. doi: 10.1152/ajpheart.1987.253.6.H1365. [DOI] [PubMed] [Google Scholar]
- Kamm K. E., Stull J. T. The function of myosin and myosin light chain kinase phosphorylation in smooth muscle. Annu Rev Pharmacol Toxicol. 1985;25:593–620. doi: 10.1146/annurev.pa.25.040185.003113. [DOI] [PubMed] [Google Scholar]
- Kitazawa T., Kobayashi S., Horiuti K., Somlyo A. V., Somlyo A. P. Receptor-coupled, permeabilized smooth muscle. Role of the phosphatidylinositol cascade, G-proteins, and modulation of the contractile response to Ca2+. J Biol Chem. 1989 Apr 5;264(10):5339–5342. [PubMed] [Google Scholar]
- Lo W. W., Fan T. P. Histamine stimulates inositol phosphate accumulation via the H1-receptor in cultured human endothelial cells. Biochem Biophys Res Commun. 1987 Oct 14;148(1):47–53. doi: 10.1016/0006-291x(87)91074-6. [DOI] [PubMed] [Google Scholar]
- Matsumoto T., Kanaide H., Nishimura J., Shogakiuchi Y., Kobayashi S., Nakamura M. Histamine activates H1-receptors to induce cytosolic free calcium transients in cultured vascular smooth muscle cells from rat aorta. Biochem Biophys Res Commun. 1986 Feb 26;135(1):172–177. doi: 10.1016/0006-291x(86)90958-7. [DOI] [PubMed] [Google Scholar]
- Morgan J. P., Morgan K. G. Stimulus-specific patterns of intracellular calcium levels in smooth muscle of ferret portal vein. J Physiol. 1984 Jun;351:155–167. doi: 10.1113/jphysiol.1984.sp015239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy R. A. Contraction in smooth muscle cells. Annu Rev Physiol. 1989;51:275–283. doi: 10.1146/annurev.ph.51.030189.001423. [DOI] [PubMed] [Google Scholar]
- Nishikawa M., Hidaka H., Adelstein R. S. Phosphorylation of smooth muscle heavy meromyosin by calcium-activated, phospholipid-dependent protein kinase. The effect on actin-activated MgATPase activity. J Biol Chem. 1983 Dec 10;258(23):14069–14072. [PubMed] [Google Scholar]
- Nishikawa M., Sellers J. R., Adelstein R. S., Hidaka H. Protein kinase C modulates in vitro phosphorylation of the smooth muscle heavy meromyosin by myosin light chain kinase. J Biol Chem. 1984 Jul 25;259(14):8808–8814. [PubMed] [Google Scholar]
- Nishikawa M., Shirakawa S., Adelstein R. S. Phosphorylation of smooth muscle myosin light chain kinase by protein kinase C. Comparative study of the phosphorylated sites. J Biol Chem. 1985 Jul 25;260(15):8978–8983. [PubMed] [Google Scholar]
- Nishimura J., Kanaide H., Miwa N., Nakamura M. Specific binding of [3H] mepyramine to histamine H1-receptors in the sarcolemma from porcine aorta and coronary artery. Biochem Biophys Res Commun. 1985 Jan 16;126(1):594–601. doi: 10.1016/0006-291x(85)90647-3. [DOI] [PubMed] [Google Scholar]
- Nishimura J., Kolber M., van Breemen C. Norepinephrine and GTP-gamma-S increase myofilament Ca2+ sensitivity in alpha-toxin permeabilized arterial smooth muscle. Biochem Biophys Res Commun. 1988 Dec 15;157(2):677–683. doi: 10.1016/s0006-291x(88)80303-6. [DOI] [PubMed] [Google Scholar]
- Nishimura J., van Breemen C. Direct regulation of smooth muscle contractile elements by second messengers. Biochem Biophys Res Commun. 1989 Sep 15;163(2):929–935. doi: 10.1016/0006-291x(89)92311-5. [DOI] [PubMed] [Google Scholar]
- Nishimura J., van Breemen C. Possible involvement of actomyosin ADP complex in regulation of Ca2+ sensitivity in alpha-toxin permeabilized smooth muscle. Biochem Biophys Res Commun. 1989 Nov 30;165(1):408–415. doi: 10.1016/0006-291x(89)91085-1. [DOI] [PubMed] [Google Scholar]
- Nishizuka Y. Perspectives on the role of protein kinase C in stimulus-response coupling. J Natl Cancer Inst. 1986 Mar;76(3):363–370. [PubMed] [Google Scholar]
- Nishizuka Y. The role of protein kinase C in cell surface signal transduction and tumour promotion. Nature. 1984 Apr 19;308(5961):693–698. doi: 10.1038/308693a0. [DOI] [PubMed] [Google Scholar]
- Rasmussen H., Forder J., Kojima I., Scriabine A. TPA-induced contraction of isolated rabbit vascular smooth muscle. Biochem Biophys Res Commun. 1984 Jul 31;122(2):776–784. doi: 10.1016/s0006-291x(84)80101-1. [DOI] [PubMed] [Google Scholar]
- Rasmussen H., Takuwa Y., Park S. Protein kinase C in the regulation of smooth muscle contraction. FASEB J. 1987 Sep;1(3):177–185. [PubMed] [Google Scholar]
- Rembold C. M., Murphy R. A. Myoplasmic [Ca2+] determines myosin phosphorylation in agonist-stimulated swine arterial smooth muscle. Circ Res. 1988 Sep;63(3):593–603. doi: 10.1161/01.res.63.3.593. [DOI] [PubMed] [Google Scholar]
- Rink T. J., Pozzan T. Using quin2 in cell suspensions. Cell Calcium. 1985 Apr;6(1-2):133–144. doi: 10.1016/0143-4160(85)90040-5. [DOI] [PubMed] [Google Scholar]
- Sakuma I., Gross S. S., Levi R. Positive inotropic effect of histamine on guinea pig left atrium: H1-receptor-induced stimulation of phosphoinositide turnover. J Pharmacol Exp Ther. 1988 Nov;247(2):466–472. [PubMed] [Google Scholar]
- Sato K., Ozaki H., Karaki H. Changes in cytosolic calcium level in vascular smooth muscle strip measured simultaneously with contraction using fluorescent calcium indicator fura 2. J Pharmacol Exp Ther. 1988 Jul;246(1):294–300. [PubMed] [Google Scholar]
- Somlyo A. V., Bond M., Somlyo A. P., Scarpa A. Inositol trisphosphate-induced calcium release and contraction in vascular smooth muscle. Proc Natl Acad Sci U S A. 1985 Aug;82(15):5231–5235. doi: 10.1073/pnas.82.15.5231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommerville L. E., Hartshorne D. J. Intracellular calcium and smooth muscle contraction. Cell Calcium. 1986 Dec;7(5-6):353–364. doi: 10.1016/0143-4160(86)90038-2. [DOI] [PubMed] [Google Scholar]
- Suematsu E., Hirata M., Hashimoto T., Kuriyama H. Inositol 1,4,5-trisphosphate releases Ca2+ from intracellular store sites in skinned single cells of porcine coronary artery. Biochem Biophys Res Commun. 1984 Apr 30;120(2):481–485. doi: 10.1016/0006-291x(84)91279-8. [DOI] [PubMed] [Google Scholar]
- Takuwa Y., Kelley G., Takuwa N., Rasmussen H. Protein phosphorylation changes in bovine carotid artery smooth muscle during contraction and relaxation. Mol Cell Endocrinol. 1988 Nov;60(1):71–86. doi: 10.1016/0303-7207(88)90121-9. [DOI] [PubMed] [Google Scholar]
- Takuwa Y., Takuwa N., Rasmussen H. Carbachol induces a rapid and sustained hydrolysis of polyphosphoinositide in bovine tracheal smooth muscle measurements of the mass of polyphosphoinositides, 1,2-diacylglycerol, and phosphatidic acid. J Biol Chem. 1986 Nov 5;261(31):14670–14675. [PubMed] [Google Scholar]
- Tsien R. Y., Pozzan T., Rink T. J. Calcium homeostasis in intact lymphocytes: cytoplasmic free calcium monitored with a new, intracellularly trapped fluorescent indicator. J Cell Biol. 1982 Aug;94(2):325–334. doi: 10.1083/jcb.94.2.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umekawa H., Naka M., Inagaki M., Onishi H., Wakabayashi T., Hidaka H. Conformational studies of myosin phosphorylated by protein kinase C. J Biol Chem. 1985 Aug 15;260(17):9833–9837. [PubMed] [Google Scholar]
- Yamamoto H., van Breemen C. Inositol-1,4,5-trisphosphate releases calcium from skinned cultured smooth muscle cells. Biochem Biophys Res Commun. 1985 Jul 16;130(1):270–274. doi: 10.1016/0006-291x(85)90412-7. [DOI] [PubMed] [Google Scholar]


