Abstract
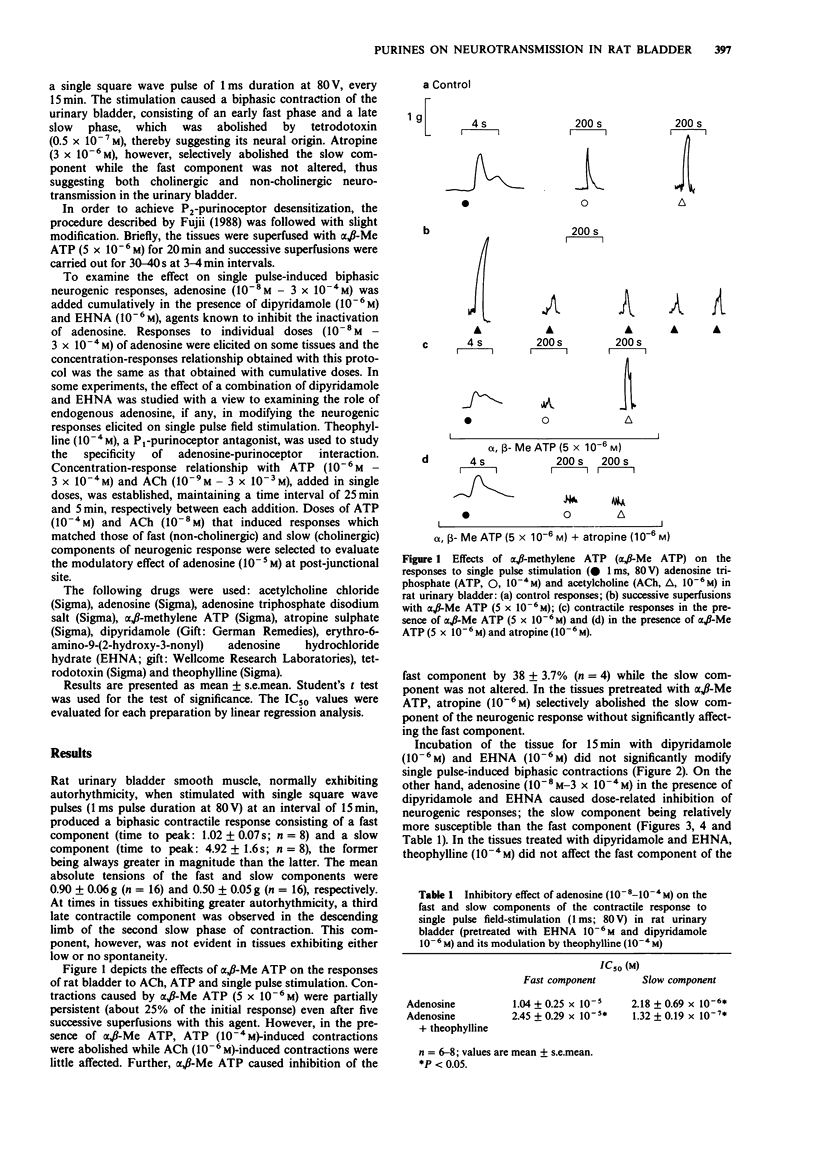
1. The effects of adenosine and alpha,beta-methylene adenosine triphosphate (alpha,beta-Me ATP) on single pulse-induced neurogenic responses and contractions caused by exogenously applied acetylcholine (ACh) and adenosine triphosphate (ATP) were examined in rat urinary bladder. 2. Application of single pulse stimulation (1 ms; 80 V) evoked a biphasic contractile response (abolished by tetrodotoxin, 0.5 x 10(-7) M) consisting of a fast (time to peak: 1.02 +/- 0.07 s) and a slow component (time to peak: 4.92 +/- 1.6 s). The selective inhibition of the slow component by atropine (3 x 10(-6) M) suggests the participation of both cholinergic and non-cholinergic neurotransmitters. 3. alpha,beta-Me ATP (5 x 10(-6) M) abolished ATP (10(-4) M)-induced contractions without altering those to ACh (10(-6) M). Further, the selective inhibition of the fast component of the neurogenic response by alpha,beta-Me ATP is suggestive of the contribution of endogenous ATP to the non-cholinergic component. 4. Adenosine (10(-8) M to 10(-4) M) caused dose-related differential inhibition of the fast (IC50, 1.04 +/- 0.25 x 10(-5) M) and slow (IC50, 2.18 +/- 0.69 x 10(-6) M) components, thereby further supporting two modes of neurotransmission in bladder. 5. Theophylline (10(-4) M) antagonized the inhibitory effects of adenosine on the non-cholinergic component, thereby implicating the participation of P1-purinoceptors in neuromodulation. In contrast, theophylline at this concentration enhanced the adenosine-induced inhibition of the cholinergic component. component.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF
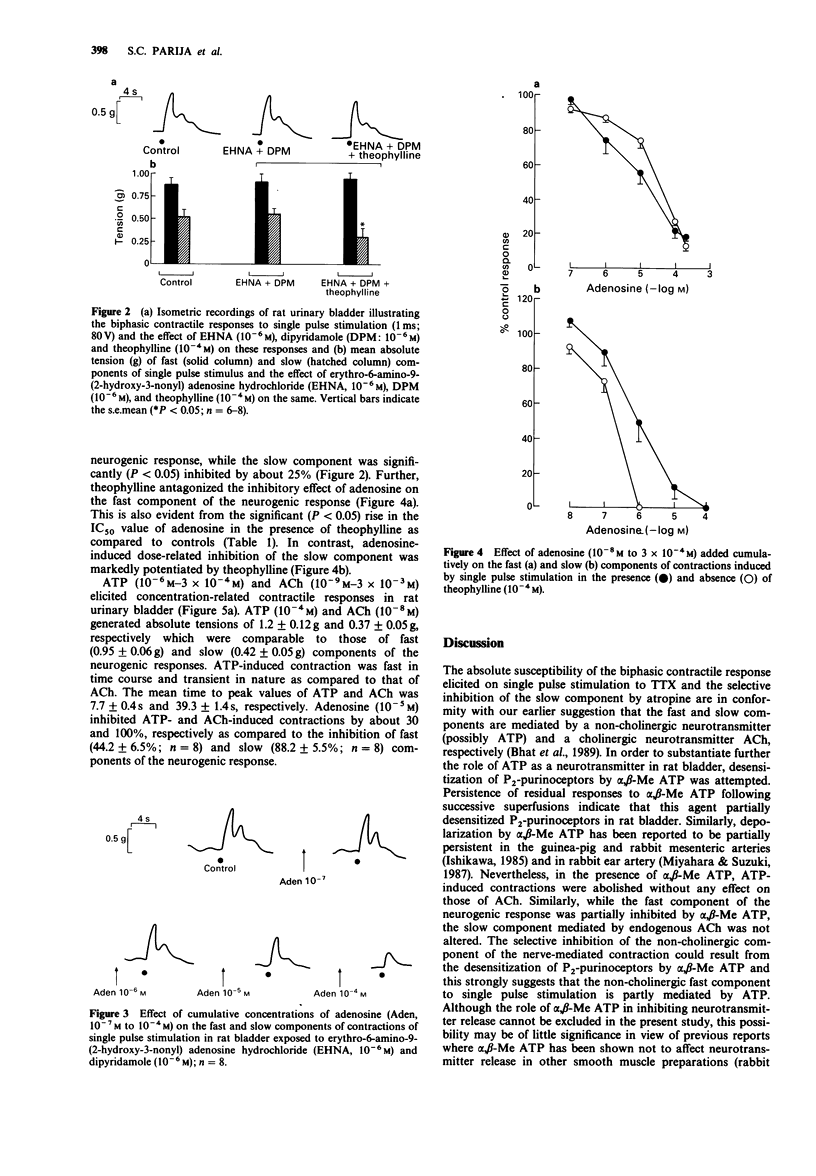
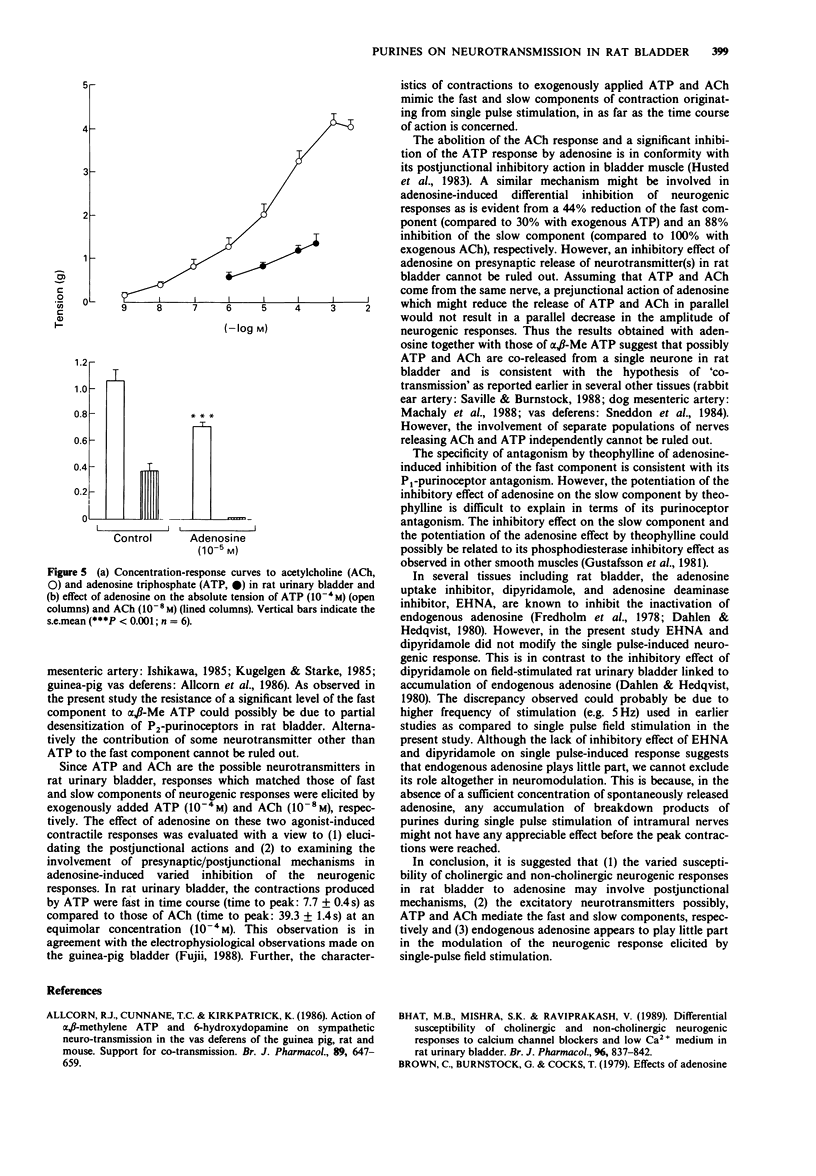

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allcorn R. J., Cunnane T. C., Kirkpatrick K. Actions of alpha, beta-methylene ATP and 6-hydroxydopamine on sympathetic neurotransmission in the vas deferens of the guinea-pig, rat and mouse: support for cotransmission. Br J Pharmacol. 1986 Dec;89(4):647–659. doi: 10.1111/j.1476-5381.1986.tb11169.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhat M. B., Mishra S. K., Raviprakash V. Differential susceptibility of cholinergic and noncholinergic neurogenic responses to calcium channel blockers and low Ca2+ medium in rat urinary bladder. Br J Pharmacol. 1989 Apr;96(4):837–842. doi: 10.1111/j.1476-5381.1989.tb11892.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown C., Burnstock G., Cocks T. Effects of adenosine 5'-triphosphate (ATP) and beta-gamma-methylene ATP on the rat urinary bladder. Br J Pharmacol. 1979 Jan;65(1):97–102. doi: 10.1111/j.1476-5381.1979.tb17337.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnstock G., Cocks T., Kasakov L., Wong H. K. Direct evidence for ATP release from non-adrenergic, non-cholinergic ("purinergic") nerves in the guinea-pig taenia coli and bladder. Eur J Pharmacol. 1978 May 15;49(2):145–149. doi: 10.1016/0014-2999(78)90070-5. [DOI] [PubMed] [Google Scholar]
- Dahlén S. E., Hedqvist P. ATP, beta-gamma-methylene-ATP, and adenosine inhibit non-cholinergic non-adrenergic transmission in rat urinary bladder. Acta Physiol Scand. 1980 Jun;109(2):137–142. doi: 10.1111/j.1748-1716.1980.tb06578.x. [DOI] [PubMed] [Google Scholar]
- Fredholm B. B., Hedqvist P., Vernet L. Effect of theophylline and other drugs on rabbit renal cyclic nucleotide phosphodiesterase, 5'-nucleotidase and adenosine deaminase. Biochem Pharmacol. 1978;27(24):2845–2850. doi: 10.1016/0006-2952(78)90199-5. [DOI] [PubMed] [Google Scholar]
- Fujii K. Evidence for adenosine triphosphate as an excitatory transmitter in guinea-pig, rabbit and pig urinary bladder. J Physiol. 1988 Oct;404:39–52. doi: 10.1113/jphysiol.1988.sp017277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustafsson L., Fredholm B. B., Hedqvist P. Theophylline interferes with the modulatory role of endogenous adenosine on cholinergic neurotransmission in guinea pig ileum. Acta Physiol Scand. 1981 Mar;111(3):269–280. doi: 10.1111/j.1748-1716.1981.tb06736.x. [DOI] [PubMed] [Google Scholar]
- Hoyle C. H., Burnstock G. Atropine-resistant excitatory junction potentials in rabbit bladder are blocked by alpha,beta-methylene ATP. Eur J Pharmacol. 1985 Aug 15;114(2):239–240. doi: 10.1016/0014-2999(85)90635-1. [DOI] [PubMed] [Google Scholar]
- Husted S., Sjögren C., Andersson K. E. Direct effects of adenosine and adenine nucleotides on isolated human urinary bladder and their influence on electrically induced contractions. J Urol. 1983 Aug;130(2):392–398. doi: 10.1016/s0022-5347(17)51175-1. [DOI] [PubMed] [Google Scholar]
- Ishikawa S. Actions of ATP and alpha, beta-methylene ATP on neuromuscular transmission and smooth muscle membrane of the rabbit and guinea-pig mesenteric arteries. Br J Pharmacol. 1985 Dec;86(4):777–787. doi: 10.1111/j.1476-5381.1985.tb11099.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasakov L., Burnstock G. The use of the slowly degradable analog, alpha, beta-methylene ATP, to produce desensitisation of the P2-purinoceptor: effect on non-adrenergic, non-cholinergic responses of the guinea-pig urinary bladder. Eur J Pharmacol. 1982 Dec 24;86(2):291–294. doi: 10.1016/0014-2999(82)90330-2. [DOI] [PubMed] [Google Scholar]
- Machaly M., Dalziel H. H., Sneddon P. Evidence for ATP as a cotransmitter in dog mesenteric artery. Eur J Pharmacol. 1988 Feb 16;147(1):83–91. doi: 10.1016/0014-2999(88)90636-x. [DOI] [PubMed] [Google Scholar]
- Miyahara H., Suzuki H. Pre- and post-junctional effects of adenosine triphosphate on noradrenergic transmission in the rabbit ear artery. J Physiol. 1987 Aug;389:423–440. doi: 10.1113/jphysiol.1987.sp016664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saville V. L., Burnstock G. Use of reserpine and 6-hydroxydopamine supports evidence for purinergic cotransmission in the rabbit ear artery. Eur J Pharmacol. 1988 Oct 18;155(3):271–277. doi: 10.1016/0014-2999(88)90513-4. [DOI] [PubMed] [Google Scholar]
- Sneddon P., Westfall D. P., Colby J., Fedan J. S. A pharmacological investigation of the biphasic nature of the contractile response of rabbit and rat vas deferens to field stimulation. Life Sci. 1984 Nov 5;35(19):1903–1912. doi: 10.1016/0024-3205(84)90470-3. [DOI] [PubMed] [Google Scholar]
- Snyder S. H. Adenosine as a neuromodulator. Annu Rev Neurosci. 1985;8:103–124. doi: 10.1146/annurev.ne.08.030185.000535. [DOI] [PubMed] [Google Scholar]
- von Kügelgen I., Starke K. Noradrenaline and adenosine triphosphate as co-transmitters of neurogenic vasoconstriction in rabbit mesenteric artery. J Physiol. 1985 Oct;367:435–455. doi: 10.1113/jphysiol.1985.sp015834. [DOI] [PMC free article] [PubMed] [Google Scholar]


