Abstract
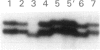
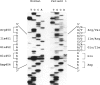
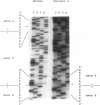
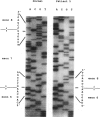
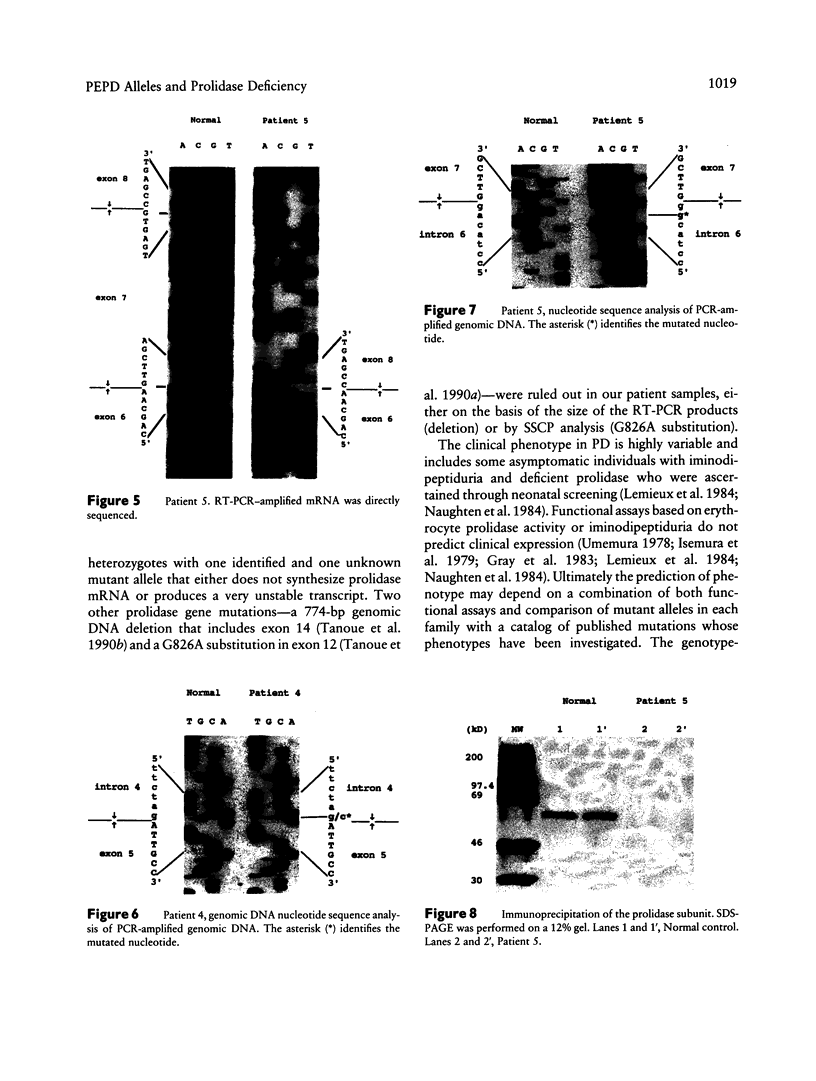
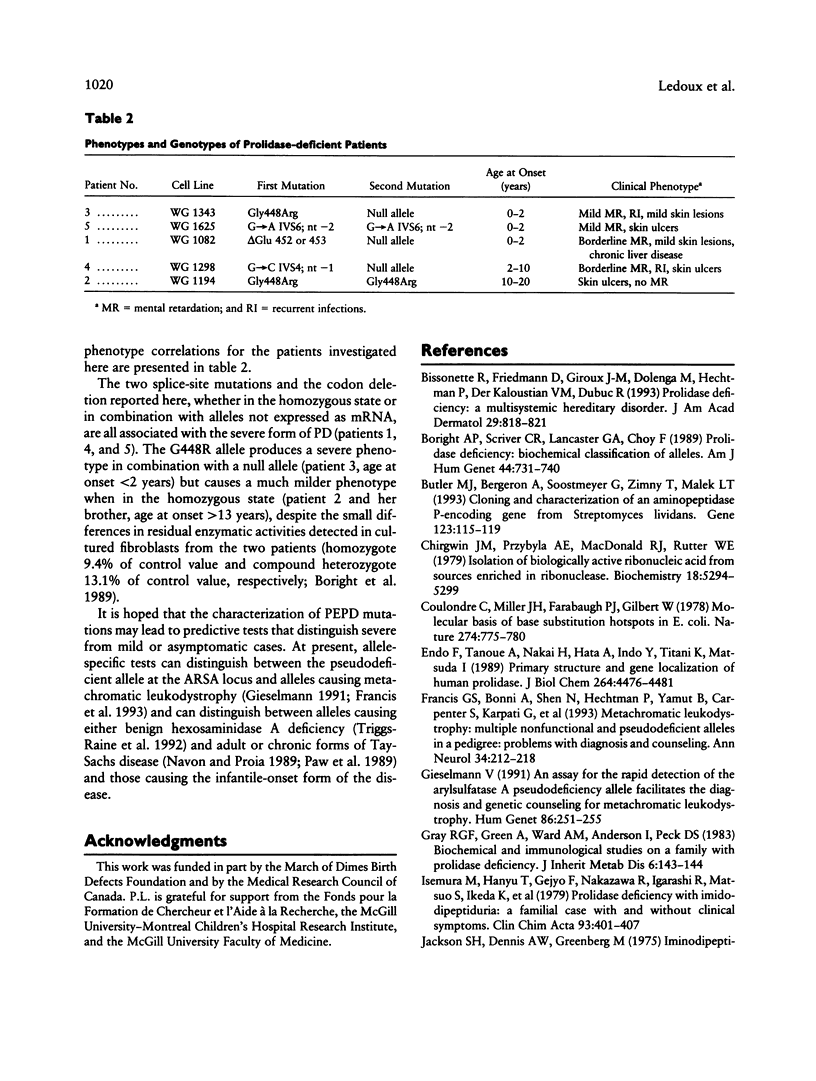
Mutations at the PEPD locus cause prolidase deficiency (McKusick 170100), a rare autosomal recessive disorder characterized by iminodipeptiduria, skin ulcers, mental retardation, and recurrent infections. Four PEPD mutations from five severely affected individuals were characterized by analysis of reverse-transcribed, PCR-amplified (RT-PCR) cDNA. We used SSCP analysis on four overlapping cDNA fragments covering the entire coding region of the PEPD gene and detected abnormal SSCP bands for the fragment spanning all or part of exons 13-15 in three of the probands. Direct sequencing of the mutant cDNAs showed a G-->A, 1342 substitution (G448R) in two patients and a 3-bp deletion (delta E452 or delta E453) in another. In the other two probands the amplified products were of reduced size. Direct sequencing of these mutant cDNAs revealed a deletion of exon 5 in one patient and of exon 7 in the other. Intronic sequences flanking exons 5 and 7 were identified using inverse PCR followed by direct sequencing. Conventional PCR and direct sequencing then established the intron-exon borders of the mutant genomic DNA revealing two splice acceptor mutations: a G-->C substitution at position -1 of intron 4 and an A-->G substitution at position -2 of intron 6. Our results indicate that the severe form of prolidase deficiency is caused by multiple PEPD alleles. In this report we attempt to begin the process of describing these alleles and cataloging their phenotypic expression.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bissonnette R., Friedmann D., Giroux J. M., Dolenga M., Hechtman P., Der Kaloustian V. M., Dubuc R. Prolidase deficiency: a multisystemic hereditary disorder. J Am Acad Dermatol. 1993 Nov;29(5 Pt 2):818–821. doi: 10.1016/0190-9622(93)70245-o. [DOI] [PubMed] [Google Scholar]
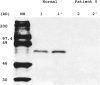
- Boright A. P., Scriver C. R., Lancaster G. A., Choy F. Prolidase deficiency: biochemical classification of alleles. Am J Hum Genet. 1989 May;44(5):731–740. [PMC free article] [PubMed] [Google Scholar]
- Butler M. J., Bergeron A., Soostmeyer G., Zimny T., Malek L. T. Cloning and characterisation of an aminopeptidase P-encoding gene from Streptomyces lividans. Gene. 1993 Jan 15;123(1):115–119. doi: 10.1016/0378-1119(93)90549-i. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Coulondre C., Miller J. H., Farabaugh P. J., Gilbert W. Molecular basis of base substitution hotspots in Escherichia coli. Nature. 1978 Aug 24;274(5673):775–780. doi: 10.1038/274775a0. [DOI] [PubMed] [Google Scholar]
- Endo F., Tanoue A., Nakai H., Hata A., Indo Y., Titani K., Matsuda I. Primary structure and gene localization of human prolidase. J Biol Chem. 1989 Mar 15;264(8):4476–4481. [PubMed] [Google Scholar]
- Francis G. S., Bonni A., Shen N., Hechtman P., Yamut B., Carpenter S., Karpati G., Chang P. L. Metachromatic leukodystrophy: multiple nonfunctional and pseudodeficiency alleles in a pedigree: problems with diagnosis and counseling. Ann Neurol. 1993 Aug;34(2):212–218. doi: 10.1002/ana.410340218. [DOI] [PubMed] [Google Scholar]
- Gieselmann V. An assay for the rapid detection of the arylsulfatase A pseudodeficiency allele facilitates diagnosis and genetic counseling for metachromatic leukodystrophy. Hum Genet. 1991 Jan;86(3):251–255. doi: 10.1007/BF00202403. [DOI] [PubMed] [Google Scholar]
- Gray R. G., Hill S. E., Pollitt R. J. Studies on the pathway from ornithine to proline in cultured skin fibroblasts with reference to the defect in hyperornithinaemia with hyperammonaemia and homocitrullinuria. J Inherit Metab Dis. 1983;6(4):143–148. doi: 10.1007/BF02310868. [DOI] [PubMed] [Google Scholar]
- Isemura M., Hanyu T., Gejyo F., Nakazawa R., Igarashi R., Matsuo S., Ikeda K., Sato Y. Prolidase deficiency with imidodipeptiduria. A familial case with and without clinical symptoms. Clin Chim Acta. 1979 May 2;93(3):401–407. doi: 10.1016/0009-8981(79)90291-2. [DOI] [PubMed] [Google Scholar]
- Jackson S. H., Dennis A. W., Greenberg M. Iminodipeptiduria: a genetic defect in recycling collagen; a method for determining prolidase in erythrocytes. Can Med Assoc J. 1975 Oct 18;113(8):759, 762-3. [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lemieux B., Auray-Blais C., Giguere R., Shapcott D. Prolidase deficiency: detection of cases by a newborn urinary screening programme. J Inherit Metab Dis. 1984;7 (Suppl 2):145–146. doi: 10.1007/978-94-009-5612-4_47. [DOI] [PubMed] [Google Scholar]
- Nakahigashi K., Inokuchi H. Nucleotide sequence between the fadB gene and the rrnA operon from Escherichia coli. Nucleic Acids Res. 1990 Nov 11;18(21):6439–6439. doi: 10.1093/nar/18.21.6439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naughten E. R., Proctor S. P., Levy H. L., Coulombe J. T., Ampola M. G. Congenital expression of prolidase defect in prolidase deficiency. Pediatr Res. 1984 Mar;18(3):259–261. doi: 10.1203/00006450-198403000-00008. [DOI] [PubMed] [Google Scholar]
- Navon R., Proia R. L. The mutations in Ashkenazi Jews with adult GM2 gangliosidosis, the adult form of Tay-Sachs disease. Science. 1989 Mar 17;243(4897):1471–1474. doi: 10.1126/science.2522679. [DOI] [PubMed] [Google Scholar]
- Ochman H., Gerber A. S., Hartl D. L. Genetic applications of an inverse polymerase chain reaction. Genetics. 1988 Nov;120(3):621–623. doi: 10.1093/genetics/120.3.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orita M., Suzuki Y., Sekiya T., Hayashi K. Rapid and sensitive detection of point mutations and DNA polymorphisms using the polymerase chain reaction. Genomics. 1989 Nov;5(4):874–879. doi: 10.1016/0888-7543(89)90129-8. [DOI] [PubMed] [Google Scholar]
- Paw B. H., Kaback M. M., Neufeld E. F. Molecular basis of adult-onset and chronic GM2 gangliosidoses in patients of Ashkenazi Jewish origin: substitution of serine for glycine at position 269 of the alpha-subunit of beta-hexosaminidase. Proc Natl Acad Sci U S A. 1989 Apr;86(7):2413–2417. doi: 10.1073/pnas.86.7.2413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proia R. L., d'Azzo A., Neufeld E. F. Association of alpha- and beta-subunits during the biosynthesis of beta-hexosaminidase in cultured human fibroblasts. J Biol Chem. 1984 Mar 10;259(5):3350–3354. [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streisinger G., Okada Y., Emrich J., Newton J., Tsugita A., Terzaghi E., Inouye M. Frameshift mutations and the genetic code. This paper is dedicated to Professor Theodosius Dobzhansky on the occasion of his 66th birthday. Cold Spring Harb Symp Quant Biol. 1966;31:77–84. doi: 10.1101/sqb.1966.031.01.014. [DOI] [PubMed] [Google Scholar]
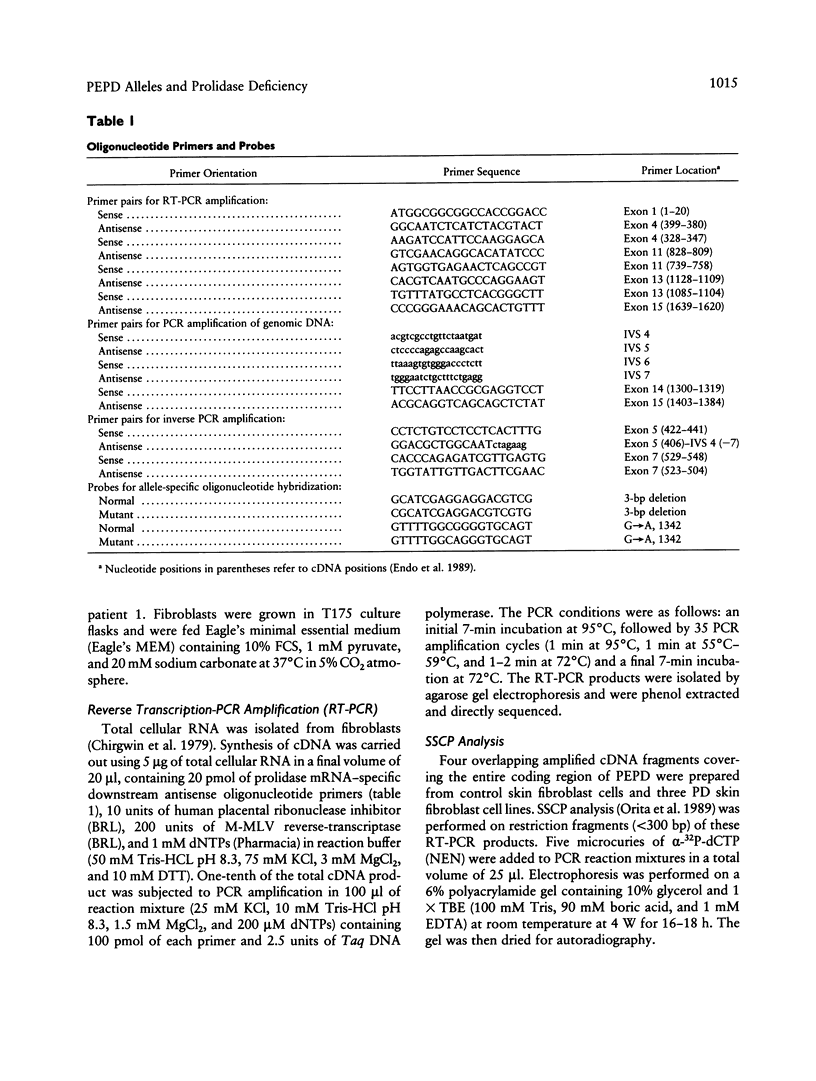
- Tanoue A., Endo F., Kitano A., Matsuda I. A single nucleotide change in the prolidase gene in fibroblasts from two patients with polypeptide positive prolidase deficiency. Expression of the mutant enzyme in NIH 3T3 cells. J Clin Invest. 1990 Jul;86(1):351–355. doi: 10.1172/JCI114708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanoue A., Endo F., Matsuda I. Structural organization of the gene for human prolidase (peptidase D) and demonstration of a partial gene deletion in a patient with prolidase deficiency. J Biol Chem. 1990 Jul 5;265(19):11306–11311. [PubMed] [Google Scholar]
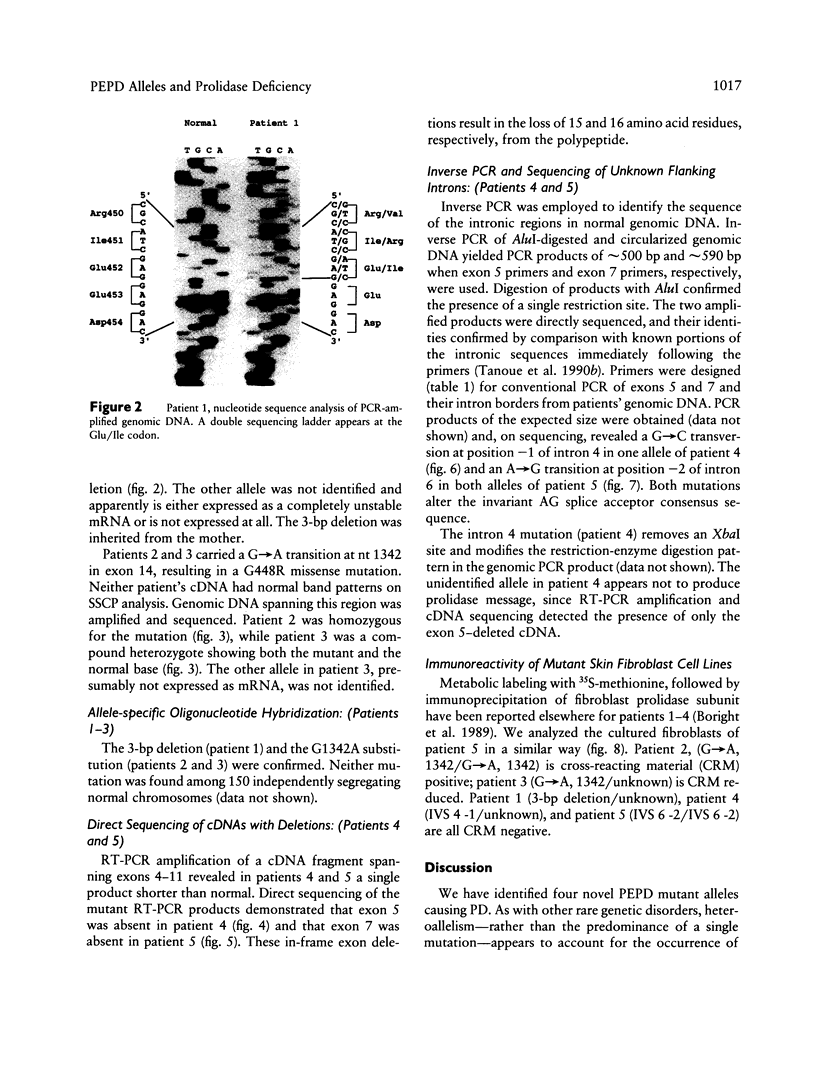
- Triggs-Raine B. L., Mules E. H., Kaback M. M., Lim-Steele J. S., Dowling C. E., Akerman B. R., Natowicz M. R., Grebner E. E., Navon R., Welch J. P. A pseudodeficiency allele common in non-Jewish Tay-Sachs carriers: implications for carrier screening. Am J Hum Genet. 1992 Oct;51(4):793–801. [PMC free article] [PubMed] [Google Scholar]
- Triglia T., Peterson M. G., Kemp D. J. A procedure for in vitro amplification of DNA segments that lie outside the boundaries of known sequences. Nucleic Acids Res. 1988 Aug 25;16(16):8186–8186. doi: 10.1093/nar/16.16.8186. [DOI] [PMC free article] [PubMed] [Google Scholar]
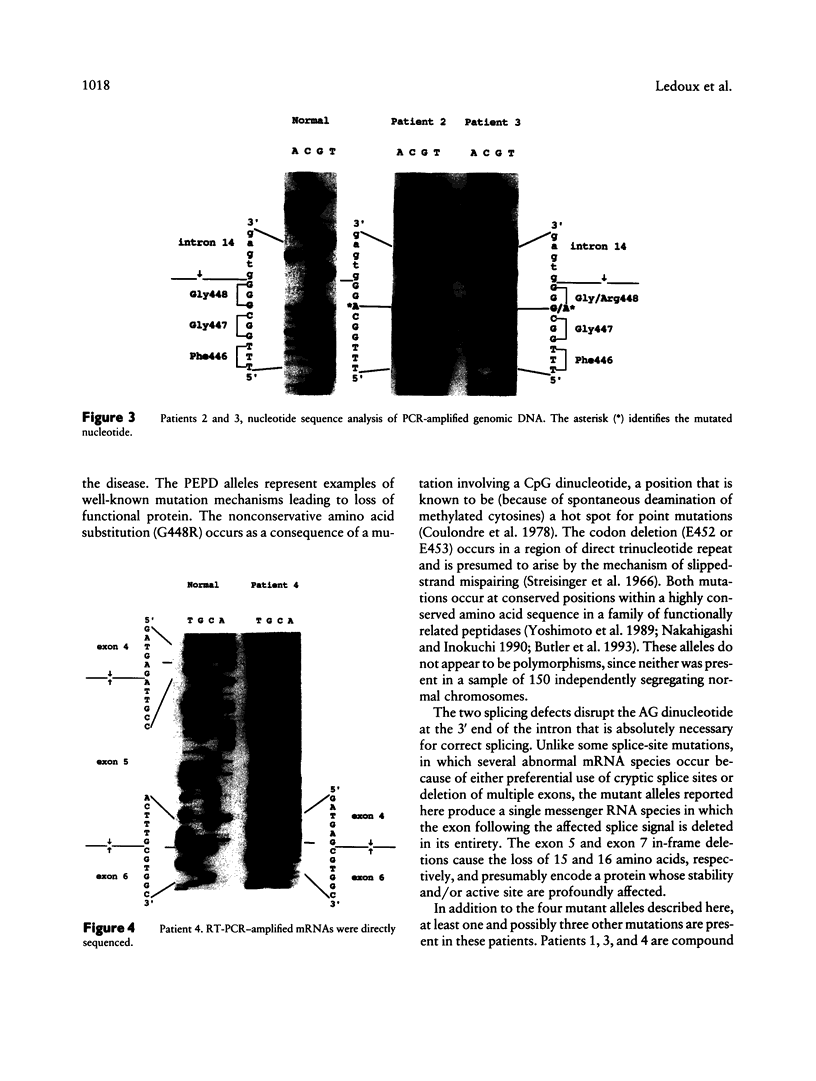
- Umemura S. Studies on a patient with iminodipeptiduria. II. Lack of prolidase activity in blood cells. Physiol Chem Phys. 1978;10(3):279–283. [PubMed] [Google Scholar]
- Yoshimoto T., Tone H., Honda T., Osatomi K., Kobayashi R., Tsuru D. Sequencing and high expression of aminopeptidase P gene from Escherichia coli HB101. J Biochem. 1989 Mar;105(3):412–416. doi: 10.1093/oxfordjournals.jbchem.a122678. [DOI] [PubMed] [Google Scholar]