Abstract
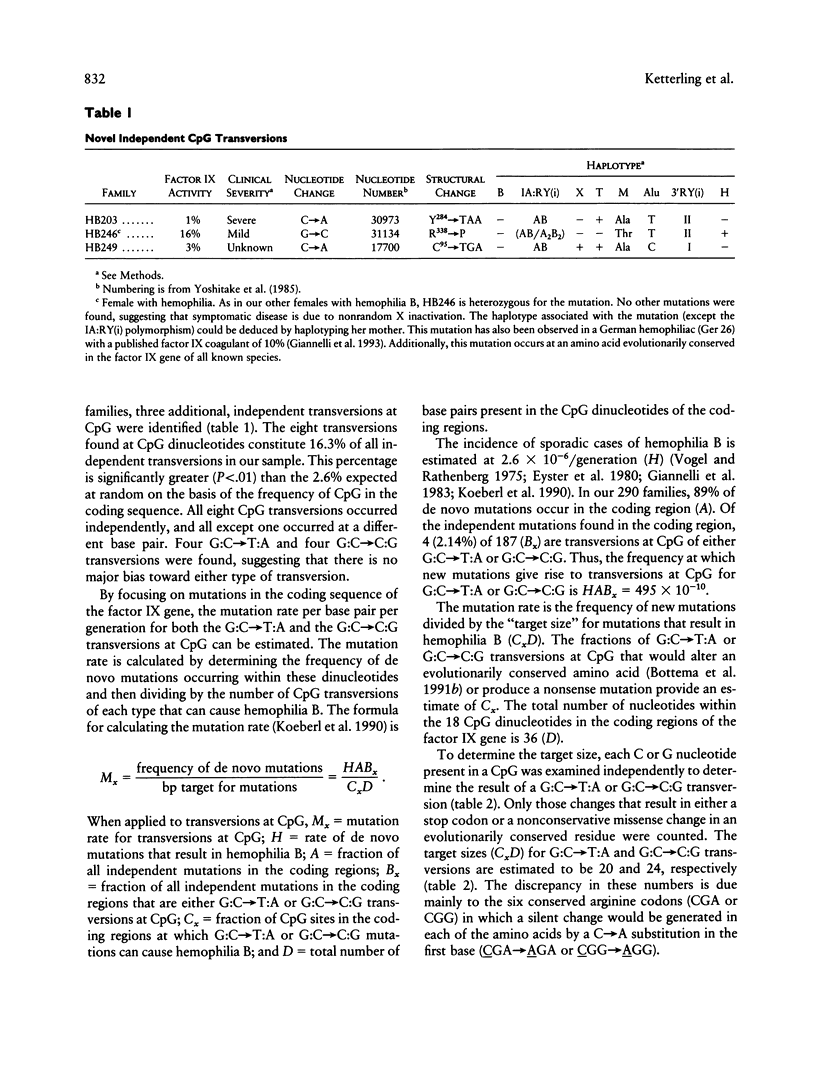
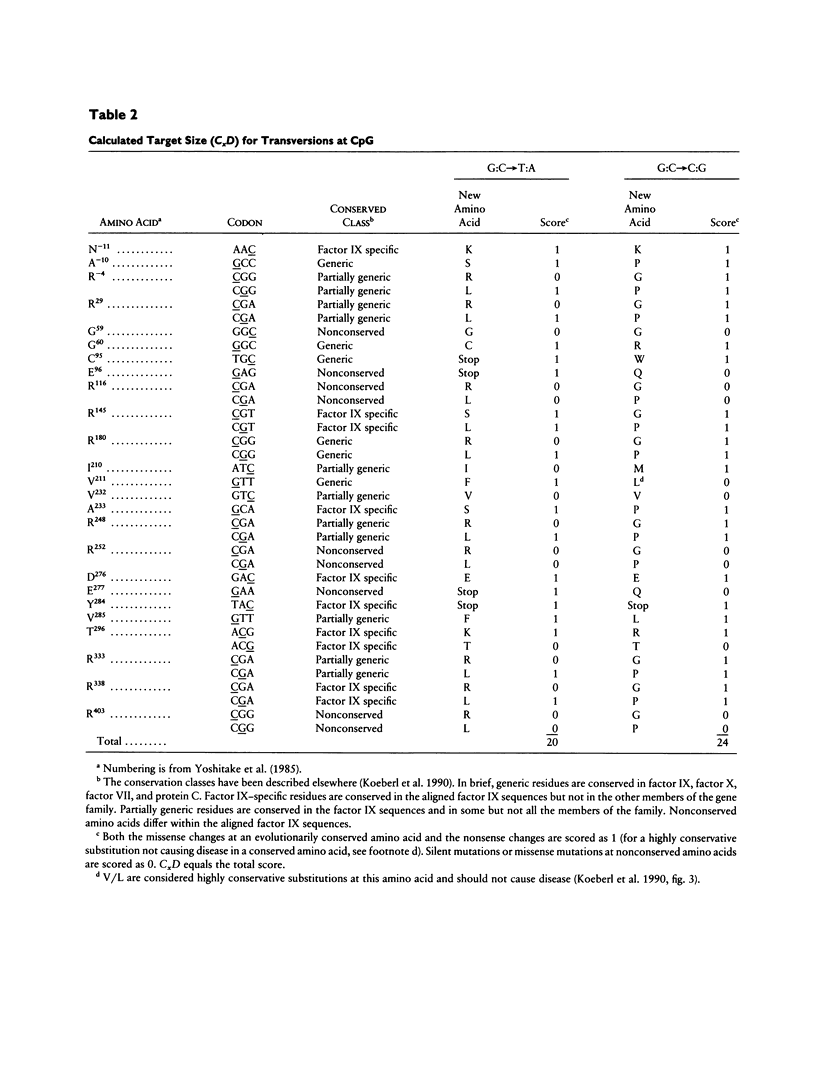
We have identified eight independent transversions at CpG in 290 consecutive families with hemophilia B. These eight transversions account for 16.3% of all independent transversions in our sample, yet the expected frequency of CpG transversions at random in the factor IX gene is only 2.6% (P < .01). The aggregate data suggest that the two types of CpG transversions (G:C-->T:A and G:C-->C:G) possess similar mutation rates (24.8 x 10(-10) and 20.6 x 10(-10), respectively), which are about fivefold greater than the comparable rates for transversions at non-CpG dinucleotides. The enhancement of transversions at CpG suggests that the model by which mutations occur at CpG may need to be reevaluated. The relationship, if any, between deamination of 5-methyl cytosine and enhancement of transversions at CpG remains to be defined.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bottema C. D., Bottema M. J., Ketterling R. P., Yoon H. S., Janco R. L., Phillips J. A., 3rd, Sommer S. S. Why does the human factor IX gene have a G + C content of 40%? Am J Hum Genet. 1991 Oct;49(4):839–850. [PMC free article] [PubMed] [Google Scholar]
- Bottema C. D., Ketterling R. P., Ii S., Yoon H. S., Phillips J. A., 3rd, Sommer S. S. Missense mutations and evolutionary conservation of amino acids: evidence that many of the amino acids in factor IX function as "spacer" elements. Am J Hum Genet. 1991 Oct;49(4):820–838. [PMC free article] [PubMed] [Google Scholar]
- Bottema C. D., Ketterling R. P., Vielhaber E., Yoon H. S., Gostout B., Jacobson D. P., Shapiro A., Sommer S. S. The pattern of spontaneous germ-line mutation: relative rates of mutation at or near CpG dinucleotides in the factor IX gene. Hum Genet. 1993 Jun;91(5):496–503. doi: 10.1007/BF00217779. [DOI] [PubMed] [Google Scholar]
- Brown T. C., Jiricny J. A specific mismatch repair event protects mammalian cells from loss of 5-methylcytosine. Cell. 1987 Sep 11;50(6):945–950. doi: 10.1016/0092-8674(87)90521-6. [DOI] [PubMed] [Google Scholar]
- Cooper D. N., Krawczak M. Cytosine methylation and the fate of CpG dinucleotides in vertebrate genomes. Hum Genet. 1989 Sep;83(2):181–188. doi: 10.1007/BF00286715. [DOI] [PubMed] [Google Scholar]
- Coulondre C., Miller J. H., Farabaugh P. J., Gilbert W. Molecular basis of base substitution hotspots in Escherichia coli. Nature. 1978 Aug 24;274(5673):775–780. doi: 10.1038/274775a0. [DOI] [PubMed] [Google Scholar]
- Dutton C. M., Bottema C. D., Sommer S. S. Alu repeats in the human factor IX gene: the rate of polymorphism is not substantially elevated. Hum Mutat. 1993;2(6):468–472. doi: 10.1002/humu.1380020607. [DOI] [PubMed] [Google Scholar]
- Eyster M. E., Lewis J. H., Shapiro S. S., Gill F., Kajani M., Prager D., Djerassi I., Rice S., Lusch C., Keller A. The Pennsylvania hemophilia program 1973-1978. Am J Hematol. 1980;9(3):277–286. doi: 10.1002/ajh.2830090306. [DOI] [PubMed] [Google Scholar]
- Giannelli F., Choo K. H., Rees D. J., Boyd Y., Rizza C. R., Brownlee G. G. Gene deletions in patients with haemophilia B and anti-factor IX antibodies. Nature. 1983 May 12;303(5913):181–182. doi: 10.1038/303181a0. [DOI] [PubMed] [Google Scholar]
- Giannelli F., Green P. M., High K. A., Sommer S., Poon M. C., Ludwig M., Schwaab R., Reitsma P. H., Goossens M., Yoshioka A. Haemophilia B: database of point mutations and short additions and deletions--fourth edition, 1993. Nucleic Acids Res. 1993 Jul 1;21(13):3075–3087. doi: 10.1093/nar/21.13.3075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustafson S., Proper J. A., Bowie E. J., Sommer S. S. Parameters affecting the yield of DNA from human blood. Anal Biochem. 1987 Sep;165(2):294–299. doi: 10.1016/0003-2697(87)90272-7. [DOI] [PubMed] [Google Scholar]
- Jacobson D. P., Schmeling P., Sommer S. S. Characterization of the patterns of polymorphism in a "cryptic repeat" reveals a novel type of hypervariable sequence. Am J Hum Genet. 1993 Aug;53(2):443–450. [PMC free article] [PubMed] [Google Scholar]
- Ketterling R. P., Bottema C. D., Koeberl D. D., Ii S., Sommer S. S. T296----M, a common mutation causing mild hemophilia B in the Amish and others: founder effect, variability in factor IX activity assays, and rapid carrier detection. Hum Genet. 1991 Jul;87(3):333–337. doi: 10.1007/BF00200915. [DOI] [PubMed] [Google Scholar]
- Ketterling R. P., Bottema C. D., Phillips J. A., 3rd, Sommer S. S. Evidence that descendants of three founders constitute about 25% of hemophilia B in the United States. Genomics. 1991 Aug;10(4):1093–1096. doi: 10.1016/0888-7543(91)90207-u. [DOI] [PubMed] [Google Scholar]
- Koeberl D. D., Bottema C. D., Ketterling R. P., Bridge P. J., Lillicrap D. P., Sommer S. S. Mutations causing hemophilia B: direct estimate of the underlying rates of spontaneous germ-line transitions, transversions, and deletions in a human gene. Am J Hum Genet. 1990 Aug;47(2):202–217. [PMC free article] [PubMed] [Google Scholar]
- Lindahl T. Instability and decay of the primary structure of DNA. Nature. 1993 Apr 22;362(6422):709–715. doi: 10.1038/362709a0. [DOI] [PubMed] [Google Scholar]
- Sarkar G., Paynton C., Sommer S. S. Segments containing alternating purine and pyrimidine dinucleotides: patterns of polymorphism in humans and prevalence throughout phylogeny. Nucleic Acids Res. 1991 Feb 11;19(3):631–636. doi: 10.1093/nar/19.3.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommer S. S. Assessing the underlying pattern of human germline mutations: lessons from the factor IX gene. FASEB J. 1992 Jul;6(10):2767–2774. doi: 10.1096/fasebj.6.10.1634040. [DOI] [PubMed] [Google Scholar]
- Stoflet E. S., Koeberl D. D., Sarkar G., Sommer S. S. Genomic amplification with transcript sequencing. Science. 1988 Jan 29;239(4839):491–494. doi: 10.1126/science.3340835. [DOI] [PubMed] [Google Scholar]
- Vogel F., Rathenberg R. Spontaneous mutation in man. Adv Hum Genet. 1975;5:223–318. doi: 10.1007/978-1-4615-9068-2_4. [DOI] [PubMed] [Google Scholar]
- Wiebauer K., Jiricny J. In vitro correction of G.T mispairs to G.C pairs in nuclear extracts from human cells. Nature. 1989 May 18;339(6221):234–236. doi: 10.1038/339234a0. [DOI] [PubMed] [Google Scholar]
- Yoshitake S., Schach B. G., Foster D. C., Davie E. W., Kurachi K. Nucleotide sequence of the gene for human factor IX (antihemophilic factor B). Biochemistry. 1985 Jul 2;24(14):3736–3750. doi: 10.1021/bi00335a049. [DOI] [PubMed] [Google Scholar]


