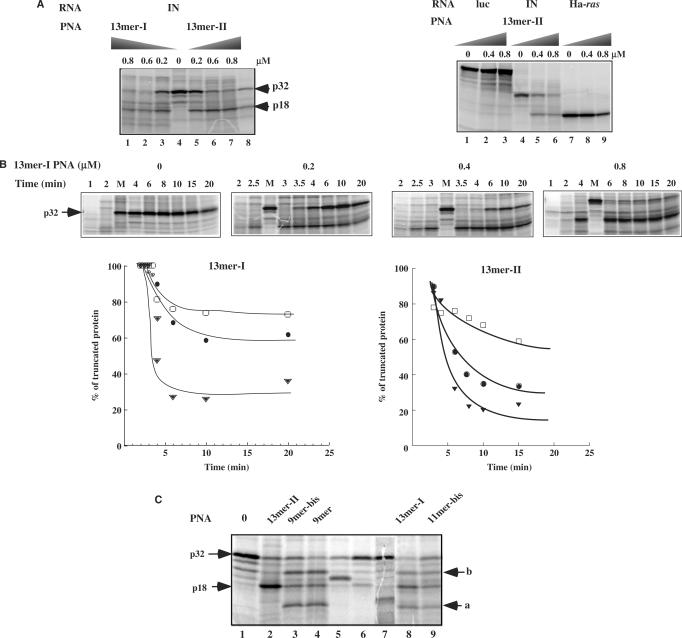
Figure 5.
(A) (Left) Activity of different PNAs (Table 1) on translation of HIV-1-integrase mRNA in a rabbit reticulocyte lysate. In vitro transcribed HIV-1-integrase mRNA was translated in the absence of PNA (0) or at the concentrations indicated above the lanes, as described in ‘Materials and Methods’ section, and subsequently analysed by SDS–PAGE on a 14% polyacrylamide gel. To determine the site of translation arrest induced by 13-mer-I and 13-mer-II PNAs, translation was carried out in the presence of a 15-mer phosphodiester ODN complementary to PPT sequence (15-mer-PPT; see Table 1 for sequences) and RNase H (E.coli) (lane 8). Arrows indicate the position of HIV-integrase protein (p32, IN) and truncated proteins (18 kDa). (Right) Control experiments: Effect of 13-mer-II PNA on translation of Ha-ras and firefly luciferase (photinus pyralis) is shown. (B) (Top) Translation kinetics was determined in the absence (0) and in the presence of different concentrations of 13-mer-I PNA (0.2, 0.4 and 0.8 μM). Lane M: 20 min of translation in the absence of PNA. (Bottom) Percentages of truncated protein synthesized in the presence of 0.2 μM (filled triangle), 0.4 μM (filled circle), 0.8 μM (open box) of 13-mer-I and 13-mer-II PNAs as a function of translation time. (C) Translation arrest experiments carried out in the presence of 0.8 μM of 13-mer-I, 13-mer-II, 9-mer, 9-mer-bis and 11-mer-bis PNAs. Arrows (a) and (b) denote the position of secondary sites of translation arrest. Lanes 6, 7, 5: polypeptide chains synthesized in the presence of RNase H and of different 15-mer phosphodiester antisense ODNs that are complementary to the PPT sequence (15-mer-PPT) and to sequences upstream (15-mer-PPT-I) and downstream (15-mer-PPT-II) the PPT site, respectively; these ODNs are targeted to potential PNA binding sites (see Table 1 for15-mers sequences).