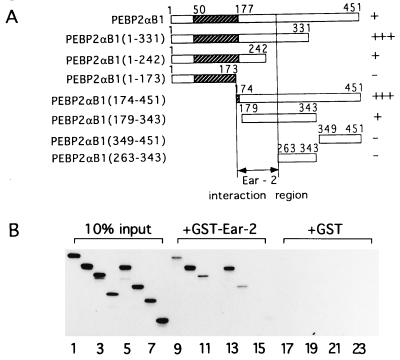
Figure 1.
Physical interaction between PEBPαB1 and Ear2. (A) Full-length PEBPαB1 and its deletion derivatives are schematically shown. Stippled boxes represent the Runt domain. Degrees of interaction shown in B are summarized as −, +, and +++ on the right. (B) PEBPαB1 and its deletion derivatives were translated by programmed reticulocyte lysate in the presence of [35S]methionine. Ten percent of the material used in the GST pull down experiment is shown (lanes 1–8). In vitro-translated proteins were mixed with GST–Ear-2-bound beads (lanes 9–16) or the GST-bound beads (lanes 17–24), and a GST pull down assay was performed. Lanes: 1, 9, and 17, PEBP2αB1; 2, 10, and 18, PEBP2αB1(1–331); 3, 11, and 19, PEBP2αB1(1–242); 4, 12, and 20, PEBP2αB1(1–173); 5, 13, and 21, PEBP2αB1(174–451); 6, 14, and 22, PEBP2αB1(179–343); 7, 15, and 23, PEBP2αB1(349–451); 8, 16, and 24, PEBP2αB1(263–343). The proteins bound to the matrix were then analyzed by SDS/PAGE on 15% gels and autoradiography.