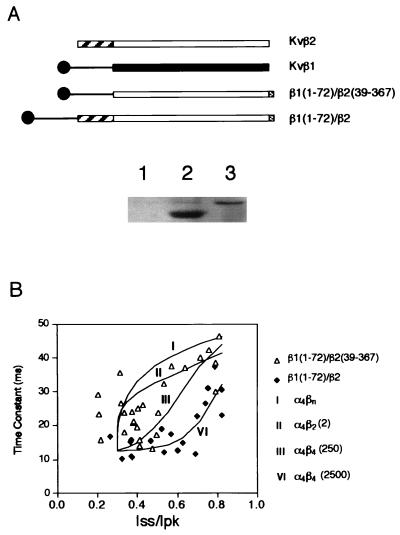
Figure 4.
Inactivation mediated by the chimeric Kvβ subunits. (A Upper) Schematic diagram showing the coding sequence of Kvβ1, Kvβ2, and two chimeric constructs. The open or solid boxes represent the core regions of either Kvβ2 or Kvβ1. The C-terminal shaded boxes show 12CA5 epitope tag. (Lower) Immunoblot showing the monoclonal antibody 12CA5 detection of chimeric β subunit protein from mock-transfected cells (lane 1), cells transfected with β1(1–72)/β2(39–367) (lane 2), and cells transfected with β1(1–72)/β2 (lane 3). (B) Inactivation time constants in milliseconds obtained from cells transfected with ShBΔ(6–46) and with chimeric Kvβ constructs were plotted against Iss/Ipk (steady-state current/peak current). ▵, Cotransfection with β1(1–72)/β2(39–367) (n = 26); ⧫, cells cotransfected with β1(1–72)/β2 (n = 19). Curves show four fitting trials representing the theoretical prediction of stoichiometry for α4βn (I), α4β2 (2) (II), and α4β4 under two conditions: α4β4 is favored by either 250-fold [α4β4(250)] (III) or 2,500-fold [α4β4(2,500)] (VI) (see Materials and Methods for details).