Figure 2.
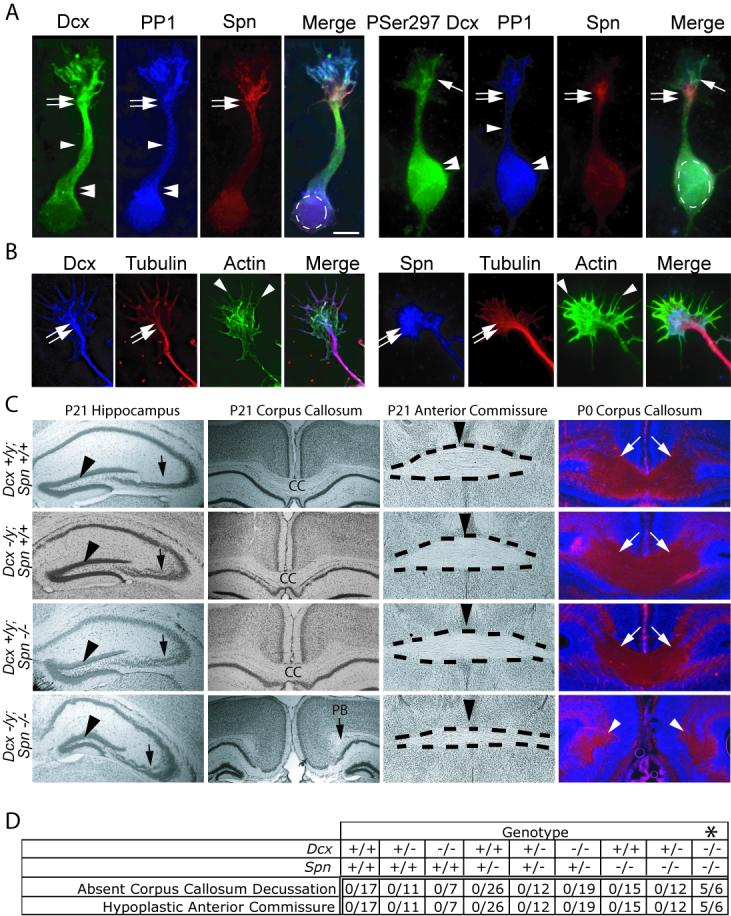
Spn and Dcx share protein distribution and co-function during brain development. (A) Dcx exhibited enrichment along MTs at the wrist (double arrows), the axonal shaft (arrowhead) and cell body (double arrowheads), PP1 was distributed diffusely, and Spn was enriched at the wrist (double arrows). PSer297 Dcx had highest expression in the growth cone (arrow) and cell body (double arrowhead). PSer297 Dcx compared with total Dcx was mostly excluded from regions of Spn localization at the wrist (double arrow), suggesting Spn may contribute to its dephosphorylation. Scale bar 10 μm. (B) Dcx and Spn overlap in distribution with the actin and MT cytoskeletons at the wrist (double arrow) and filopodia (arrowheads). (C) Dcx and Spn cooperate in axonal outgrowth of multiple long distance projections. Both Spn −/− and Dcx −/y showed defective lamination of the CA3 region (arrow), whereas Spn −/−; Dcx −/y (DKO) showed possibly worsened defect compared with single knockouts. The granule cell layer was unaffected (arrowhead). The CC decussation was evident in all but the DKO, where it was replaced by Probst bundles (PB). The anterior commissure (dashes) showed normal appearance in all but the DKO where it was hypoplastic. Midline indicated by arrowhead. At P0, decussating CC fibers (stained with L1CAM, arrows) were visible in all but the DKO, where they terminated in Probst bundles (arrowheads). (D) Expressivity of ACC and hypoplastic anterior commissure among offspring from 20 litters of double heterozygous matings. Number of mice with each phenotype over total of each genotype are listed. Note that none of the mice except the Spn −/− ; Dcx −/− (DKO) showed ACC and hypoplastic anterior commissure phenotype. Dcx −/− entries include both −/− females and −/y male null mice. * = p < 0.001, Chi squared test.
