Figure 5.
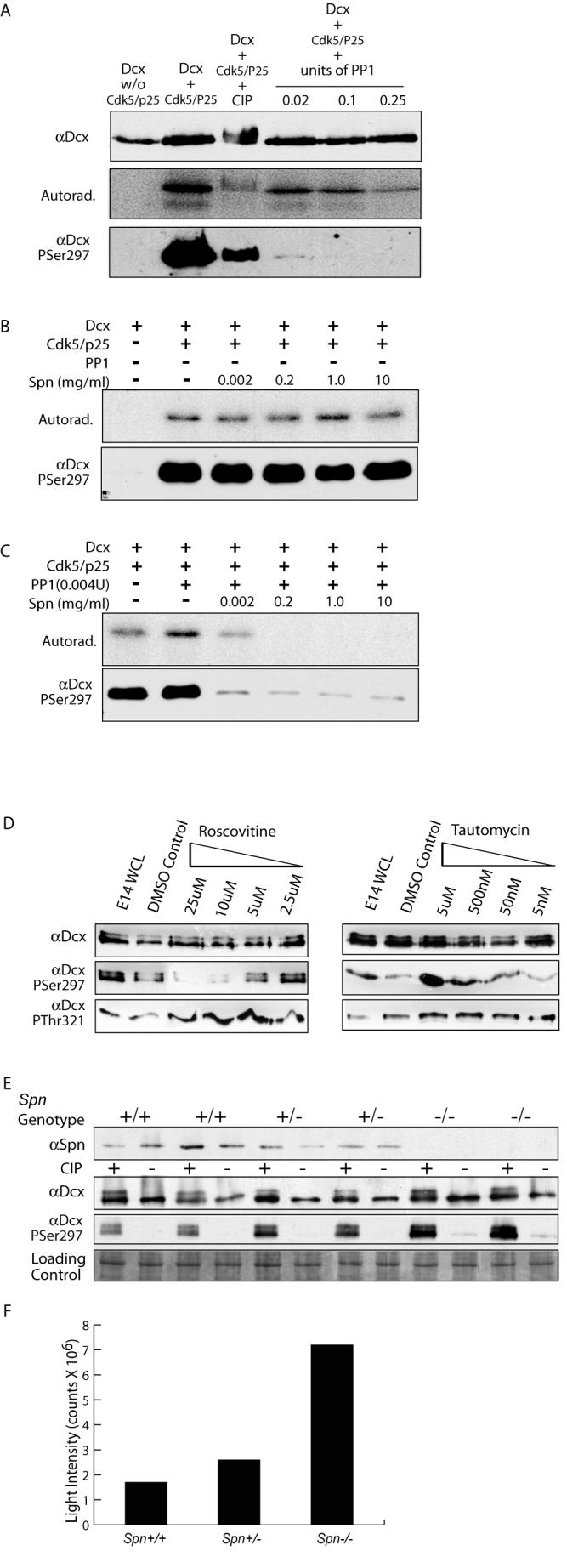
Spn required for PP1-mediated dephosphorylation of PSer297 Dcx. (A) PP1 at high unit concentrations was capable of dephosphorylating Dcx at PSer297, based on autoradiogram or immunoreactivity with αPSer297 following [32P] incorporation. PP1 was a more specific phosphatase for the PSer297 site than CIP, resulting in nearly complete dephosphorylation at all concentrations tested. (B) Spn alone has no effect on [32P] retention or αPSer297 reactivity. (C) Low levels of PP1 (5X lower than used in (A) in the absence of Spn had no effect on [32P] retention or αPSer297 reactivity but increasing amounts of Spn promoted dephosphorylation of Dcx by PP1. (D) PP1 and Cdk5 act in opposing fashions to modulate phosphorylation state of Dcx Ser297 in cortical neurons. Cortical neurons with increasing roscovitine (inhibits Cdk5) or tautomycin (inhibits PP1) were analyzed by Western with Dcx PSer297 and PThr321 antibodies. Roscovitine blocked and tautomycin enhanced PSer297 reactivity but not PThr321. (E) Brain lysates from E16 littermates showed increased PSer297 reactivity as Spn dosage was decreased. (F) Quantification of PSer297 Dcx band intensity standardized to control shows a four-fold increase in reactivity in Spn −/− versus +/+.
