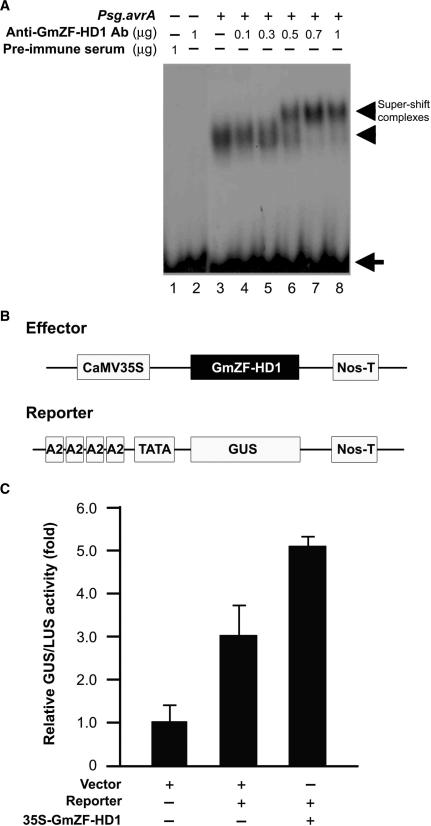
Figure 8.
Direct interaction with and activation of the GmCaM4 promoter by the GmZF-HD1 protein. (A) Gel mobility super-shift assay using an anti-GmZF-HD1 antibody. Various concentrations of polyclonal antibodies raised against the GST::GmZF-HD1 protein were incubated with the protein–DNA complex formed with the 32P-labeled 30-nt fragment of GmCaM-4 promoter and nuclear extracts from pathogen-treated soybean suspension culture cells. Lane 1 contains preimmune serum, lane 2 contains 1 μg affinity-purified GmZF-HD1 antibody, and lane 3 contains nuclear extracts alone. Nuclear extracts from pathogen-treated cells were preincubated with 0.1 μg (lane 4), 0.3 μg (lane 5), 0.5 μg (lane 6), 0.7 ug (lane 7) or 1 μg (lane 8) affinity-purified GmZF-HD1 antibody before adding the 32P-labeled 30-nt fragment of GmCaM-4 promoter as a probe. Free (arrow) and protein-complexed (arrowhead) probes were separated on an 8% polyacrylamide gel and visualized by autoradiography. (B) Schematic representation of the effector and reporter constructs used in transient expression assays in Arabidopsis protoplasts. In the effector construct, GmZF-HD1 cDNA was inserted between the CaMV 35S promoter and a Nos terminator (Nos-T). In the reporter construct, four tandem repeats of the A2 region containing the 30-nt fragment of the GmCaM-4 promoter were subcloned upstream of a minimal TATA promoter-GUS construct. (C) Effect of the GmZF-HD1 protein on GmCaM-4 promoter activity in Arabidopsis protoplasts. The empty vector without GmZF-HD1 and the A2 region of the GmCaM-4 promoter were used as controls. For normalization of transformation efficiency, the CaMV 35S promoter-luciferase (LUC) plasmid was co-transfected in each experiment. The data are presented as mean ± SE of results from three independent samples. The numbers show the relative increase in expression compared to the value obtained with the vector control.